Maprotiline
 | |
 | |
| Names | |
|---|---|
| Trade names | Ludiomil, Maprolu, others |
| Other names | Maprotiline hydrochloride; maprotiline methanesulfonate; Ba 34276[1][2][3] |
| |
| Clinical data | |
| Drug class | Tetracyclic antidepressant (TeCA)[4] |
| Main uses | Depression, anxiety, bipolar disorder[4] |
| Side effects | Dry mouth, constipation, sleepiness, dizziness, anxiety[4] |
| Pregnancy category |
|
| Routes of use | By mouth, intramuscular, intravenous |
| Onset of action | 6 hours |
| Typical dose | 75 mg to 150 mg/day[4] |
| External links | |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a682158 |
| Legal | |
| Legal status | |
| Pharmacokinetics | |
| Bioavailability | 66–70% |
| Protein binding | 88% |
| Metabolism | Liver |
| Elimination half-life | 27–58 hours |
| Excretion | Urine (57%) and bile (30%) as glucuronides, 3–4% as unchanged drug |
| Chemical and physical data | |
| Formula | C20H23N |
| Molar mass | 277.411 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Maprotiline, sold under the brand name Ludiomil among others, is a medication used to treat depression, anxiety, and bipolar disorder.[4] It is taken by mouth.[4]
Common side effects include dry mouth, constipation, sleepiness, dizziness, and anxiety.[4] Other side effects may include suicide, rash, seizures, bipolar, and arrhythmias.[4] While there is no clear harm from us in pregnancy, such use has not been well studied.[5] It is a tetracyclic antidepressant (TeCA).[4]
Maprotiline was approved for medical use in the United States in 1980.[4] In the United States 100 tablets of 75 mg costs about 175 USD as of 2021.[6] It is available is various countries globally.[2]
Medical uses
Maprotiline is used in the treatment of depression, such as depression associated with agitation or anxiety and has similar efficacy to the antidepressant drug moclobemide.[7]
- Treatment of depression of all forms and severities (endogenous, psychotic, involutional, and neurotic) especially for depression associated with agitation or anxiety
- Panic disorder
- Neuropathic pain
- Treatment of the depressive phase in bipolar depression
- For the symptomatic relief of anxiety, tension or insomnia
The use of maprotiline in the treatment of enuresis in pediatric patients has so far not been systematically explored and its use is not recommended.[8] Safety and effectiveness in the pediatric population in general have not been established. Anyone considering the use of maprotiline in a child or adolescent must balance the potential risks with the clinical need. In general, lower dosages are recommended for patients over 60 years of age. Dosages of 50 mg to 75 mg daily are usually satisfactory as maintenance therapy for elderly patients who do not tolerate higher amounts.[9][10]
Dosage
It is generally take as 75 mg per day though may be increased up to 225 mg per day.[4]
- Coated Tablets, 10 mg, 25 mg, 50 mg, and 75 mg
- Injectable concentrate, 25 mg
Contraindications
Maprotiline may worsen psychotic conditions like schizophrenia and should be given with caution. The antipsychotic treatment should be continued. Patients with bipolar affective disorder should not receive antidepressants whilst in a manic phase, as antidepressants can worsen mania.
Absolute
- Hypersensitivity to maprotiline or to other TCAs and TeCAs
- Hypertrophy of the prostate gland with urine hesitancy
- Closed angle glaucoma
Caution
- Concomitant treatment with a MAO inhibitor
- Serious impairment of liver and kidney function
- Epilepsy and other conditions that lower the seizure threshold (active brain tumors, alcohol withdrawal, other medications)
- Serious cardiovascular conditions (arrhythmias, heart insufficience, state after myocardial infarction etc.)
- Treatment of patients under age 18[11]
Side effects
The side-effect profile is comparable to other TCAs and TeCAS and many of the following are due to anticholinergic (which are less prominent than those of most TCAs) and antihistamine effects.[8] Most often seen are:
- Dizziness
- Drowsiness
- Somnolence
- Fatigue
- Dry mouth (and complications of long-term uncontrolled dry mouth such as dental caries)
- Constipation
- Vertigo
- Nausea (rare, incidence of ~2%) and vomiting
- Increased appetite and weight gain
- Orthostatic hypotension, hypertension, sinus tachycardia, heart-block, arrhythmias and other cardiac effects
- Sexual dysfunction in men: impotence, priapism, delayed ejaculation, anejaculation, decreased libido
- Sexual dysfunction in women: decreased libido, vaginal dryness, painful sexual intercourse, anorgasmia
- Allergic skin reactions such as rash or urticaria (more often than with other antidepressants). Rarely, severe skin reactions such as erythema multiforme can occur.
- Photosensitivity
- Agitation, confusion
- Induction of hypomania or mania in patients suffering from underlying bipolar affective disorder
- Psychotic symptoms
- Tremor
- Extrapyramidal symptoms
- Headache
- Seizures (at high doses)
- Rare haematological complications: leukopenia and agranulocytosis (dangerous fall in white blood cells)
- Fever
- Urinary retention
Maprotiline causes a strong initial sedation (first 2 to 3 weeks of therapy) and is therefore indicated to treat agitated patients or those with suicidal risks. It causes anticholinergic side effects (dry mouth, constipation, confusion, tachycardia) with a lower incidence than amitriptyline. Originally, the manufacturer claimed that maprotiline is better tolerated than other TCAs and TeCAs. However, seizures, leukopenia and skin reactions occur more often with maprotiline than with comparable drugs like amitriptyline.
Maprotiline has no known potential for abuse and psychological dependence.
Withdrawal
Withdrawal symptoms frequently seen when treatment with maprotiline is stopped abruptly (agitation, anxiety, insomnia, sometimes activation of mania or rebound depression) are not indicative of addiction and can be avoided by reducing the daily dose of maprotiline gradually by approximately 25% each week. If treatment has to be stopped at once due to medical reasons, the use of a benzodiazepine (e.g. lorazepam, clonazepam, or alprazolam) for a maximum of 4 weeks as needed will usually suppress withdrawal symptoms.
Suicide
Same as other antidepressants, maprotiline increased the risk compared to placebo of suicidal thinking and behavior (suicidality) in children, adolescents and young adults in short-term studies of major depressive disorder (MDD) and other psychiatric disorders. Anyone considering the use of maprotiline or any other antidepressant in a child, adolescent, or young adult must balance this risk with the clinical need. Short-term studies did not show an increase in the risk of suicidality with antidepressants compared to placebo in adults beyond age 24; there was a reduction in risk with antidepressants compared to placebo in adults aged 65 and older. Depression and certain other psychiatric disorders are themselves associated with increases in the risk of suicide. Patients of all ages who are started on antidepressant therapy should be monitored appropriately and observed closely for clinical worsening, suicidality, or unusual changes in behavior. Families and caregivers should be advised of the need for close observation and communication with the prescriber. Maprotiline is not approved for use in pediatric patients.[12]
Pregnancy and breastfeeding
Reproduction studies have been performed in female laboratory rabbits, mice, and rats at doses up to 1.3, 7, and 9 times the maximum daily human dose respectively and have revealed no evidence of impaired fertility or harm to the fetus due to maprotiline. There are, however, no adequate and well controlled studies in pregnant women. Because animal reproduction studies are not always predictive of human response, this drug should be used during pregnancy only if clearly needed.
Maprotiline is excreted in breast milk. At steady-state, the concentrations in milk correspond closely to the concentrations in whole blood. Caution should be exercised when maprotiline hydrochloride is administered to a nursing woman.
Interactions
Maprotiline has a wide range of possible interactions. Some are typical for TCAs and TeCAs, others are caused by specific metabolic effects (e.g. high plasma-protein-binding) of maprotiline:
- Irreversible MAO-inhibitors: agitation, delirium, coma, hyperpyrexia (high fever), seizures and severe changes in blood pressure. Treatment-resistant and hospitalized patients may be treated concomitantly with an MAO-inhibitor, if they are closely monitored and if the initial dose of the MAO-Inhibitor is low.
Increased drug actions:
- Other antidepressants, barbiturates, narcotics, sedating antihistamines, anticonvulsive drugs, alcohol - resulting in increased central depression
- Anticholinergics (antiparkinsonian agents, TCAs and TeCAs) - resulting in increased anticholinergic action (dry mouth, constipation etc.)
- Sympathomimetics (also those used in local anesthetics like noradrenaline) : sympathomimetic effects increased (increased blood pressure, pulse rate, paleness of skin etc.)
- Nitrates and antihypertensives (e.g. beta-blockers) - increased antihypertensive action with pronounced fall in blood pressure
Decreased drug actions:
- Guanethidine, Reserpine, Guanfacine : antihypertensive effects decreased
- Clonidine : antihypertensive effects decreased and risk of (massive) rebound hypertension.
Other types of interaction:
- Drugs, which induce certain enzymes in the liver, e.g. barbiturates, phenytoin, carbamazepine and oral anticonceptive drugs, enhance the elimination of maprotiline and decrease its antidepressant effects. Additionally the blood-concentrations of phenytoin or carbamazepine may be increased, leading to a higher incidents of side effects.
- The concomitant use of maprotiline and neuroleptics can lead to increased maprotiline blood-levels and to seizures. Combining maprotiline and thioridazine could induce severe arrhythmias.
- Additionally, increased blood-levels of Maprotiline are possible, if certain beta-blocking agents (e.g. Propranolol) are given concomitantly.
- Maprotiline may amplify the actions of coumarin-type anticoagulants (e.g. warfarin, phenprocoumon). The plasma-prothrombin-activity must be assessed closely in order to avoid overt bleedings.
- Maprotiline can increase the actions of oral antidiabetic drugs (sulfonylureas) and Insulin. Diabetic patients should have regular assessments of their blood-glucose-levels.
- The concomitant application with fluoxetine or fluvoxamine may lead to significantly increased plasma-levels of maprotiline with a high incidence of maprotiline side effects. Due to the long half-lives of fluoxetine and fluvoxamine this effect may persist.
Pharmacology
It may alternatively be classified as a tricyclic antidepressant (TCA), specifically a secondary amine.[13] In terms of its chemistry and pharmacology, maprotiline is closely related to other secondary amine TCAs like nortriptyline and protriptyline, and has similar effects to them.[14][13]
Pharmacodynamics
| Site | Ki (nM) | Species | Ref | |
|---|---|---|---|---|
| SERT | 5,800 | Human | [16] | |
| NET | 11–12 | Human | [16][17] | |
| DAT | 1,000 | Human | [16] | |
| 5-HT2A | 51 | Rat | [18] | |
| 5-HT2C | 122 | Rat | [18] | |
| 5-HT6 | ND | ND | ND | |
| 5-HT7 | 50 | Guinea pig | [19] | |
| α1 | 90 | Human | [20] | |
| α2 | 9,400 | Human | [20] | |
| D1 | 402 | Human | [21] | |
| D2 | 350–665 | Human | [21][20] | |
| D3 | 504 | Human | [21] | |
| D4 | ND | ND | ND | |
| D5 | 429 | Human | [21] | |
| H1 | 0.79–2.0 | Human | [22][21][23][20] | |
| H2 | 776 | Human | [22] | |
| H3 | 66,100 | Human | [21] | |
| H4 | 85,100 | Human | [22] | |
| mACh | 570 | Human | [24][20] | |
| Values are Ki (nM). The smaller the value, the more strongly the drug binds to the site. | ||||
Maprotiline exhibits strong effects as a norepinephrine reuptake inhibitor with only weak actions the reuptake of serotonin and dopamine.[25][8] It is also a strong antagonist of the H1 receptor, a moderate antagonist of the 5-HT2 and α1-adrenergic receptors, and a weak antagonist of the D2 and muscarinic acetylcholine receptors. Maprotiline has also more recently been identified as a potent antagonist of the 5-HT7 receptor, with this action potentially playing an important role in its antidepressant effectiveness.[26] The drug is a strong antihistamine, but unlike most TCAs, has minimal anticholinergic effects.[27]
The pharmacological profile of maprotiline explains its antidepressant, sedative, anxiolytic, and sympathomimetic activities. In accordance to the pharmacological characteristics it is used in the treatment of depression, such as depression associated with agitation or anxiety. Additionally, it shows strong antagonism against reserpine-induced effects in animal studies, as do the other 'classical' antidepressants. Although maprotiline behaves in most regards as a 'first-generation antidepressant' it is commonly referred to as 'second-generation antidepressant'.
The postulated mechanism of maprotiline is that it acts primarily by potentiation of central adrenergic synapses by blocking reuptake of norepinephrine at nerve endings. This pharmacological action is thought to be primarily responsible for the drug's antidepressant and anxiolytic effects. It is a strong norepinephrine reuptake inhibitor with only weak effects on serotonin and dopamine reuptake. At higher doses however, maprotiline increases serotonergic transmission and increases the level of serotonin available.[28]
Pharmacokinetics
After oral use absorption is good. It binds to plasma proteins 80–90%. Maximal plasma concentration is reached 6 hours after use. The mean time to peak is 12 hours. The terminal half-life of averages 51 hours.
Chemistry


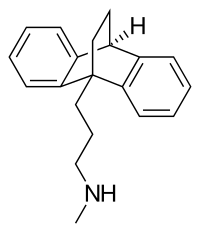
Maprotiline is a tetracyclic compound and is grouped with the TeCAs.[14][13] Its chemical name is N-methyl-9,10-ethanoanthracen-9(10H)-propylamine.[29] The drug has a dibenzobicyclo[2.2.2]octadiene (9,10-dihydro-9,10-ethanoanthracene) ring system; that is, a tricyclic anthracene ring system with an ethylene bridge across the central ring.[14][13] This results in it having a unique three-dimensional central ring (a bicyclo[2.2.2]octane or 1,4-endoethylenecyclohexane ring) and being a tetracyclic rather than a tricyclic compound.[14] However, it could also or alternatively be considered to be a tricyclic and hence a TCA.[13] In addition to its heterocyclic ring system, maprotiline has an alkylamine side chain attached similarly to other TCAs (but notably unlike other TeCAs).[14][13] In terms of the side chain, it is a secondary amine,[13] and its chemical structure, aside from the ethylene link in the central ring, is similar to that of secondary amine TCAs like nortriptyline and protriptyline.[14][29] In accordance, the pharmacology of maprotiline is very similar to that of secondary amine TCAs.[14][13]
Maprotiline is very similar in structure to the anxiolytic, sedative, and muscle relaxant drug benzoctamine (Tacitin).[14][30] The only structural difference between the two compounds is in the length of their side chain.[14][30] However, this modification results in considerable differences in their pharmacological and therapeutic effects.[14][30]
History
Maprotiline was developed by Ciba (now operated by Novartis).[31] It was patented in 1966 and was first described in the literature in 1969.[31] The drug was introduced for medical use in 1974.[31][32] Generics are now widely available. It was introduced after most of the other TCAs but was the first TeCA to be developed and marketed, with the TeCAs mianserin and amoxapine following shortly thereafter and mirtazapine being introduced later on.[31][32]
Society and culture

Generic names
Maprotiline is the English and French generic name of the drug and its INN, USAN, BAN, and DCF, while maprotiline hydrochloride is its USAN, USP, BANM and JAN.[1][2][33][3] Its generic name in Spanish and Italian and its DCIT are maprotilina, in German is maprotilin, and in Latin is maprotilinum.[2][3] The methanesulfonate (mesylate) salt is known unofficially as maprotiline methanesulfonate.[2][3]
Brand names
Maprotiline is marketed throughout the world mainly under the brand name Ludiomil.[2][3] It is also available under a variety of other brand names including Deprilept, Maprolu, and Psymion among others.[2][3]
References
- ↑ 1.0 1.1 J. Elks (14 November 2014). The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. pp. 752–. ISBN 978-1-4757-2085-3.
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 2.6 Index Nominum 2000: International Drug Directory. Taylor & Francis. 2000. pp. 630–. ISBN 978-3-88763-075-1.
- ↑ 3.0 3.1 3.2 3.3 3.4 3.5 "Maprotiline - Drugs.com". drugs.com. Archived from the original on 16 August 2017. Retrieved 28 March 2018.
- ↑ 4.00 4.01 4.02 4.03 4.04 4.05 4.06 4.07 4.08 4.09 4.10 "Maprotiline Monograph for Professionals". Drugs.com. Archived from the original on 19 January 2021. Retrieved 14 November 2021.
- ↑ "Maprotiline (Ludiomil) Use During Pregnancy". Drugs.com. Archived from the original on 5 December 2020. Retrieved 14 November 2021.
- ↑ "Maprotiline Prices, Coupons & Patient Assistance Programs". Drugs.com. Archived from the original on 10 August 2020. Retrieved 14 November 2021.
- ↑ Delini-Stula A, Mikkelsen H, Angst J (October 1995). "Therapeutic efficacy of antidepressants in agitated anxious depression--a meta-analysis of moclobemide studies". J Affect Disord. 35 (1–2): 21–30. doi:10.1016/0165-0327(95)00034-K. PMID 8557884.
- ↑ 8.0 8.1 8.2 "DRUGDEX Evaluations - Maprotiline". Archived from the original on 31 December 2019. Retrieved 25 April 2013.
- ↑ https://www.nlm.nih.gov/medlineplus/druginfo/meds/a682158.html Archived 2016-07-05 at the Wayback Machine. Retrieved 29 September 2013.
- ↑ https://www.drugs.com/pro/maprotiline.html Archived 2019-12-30 at the Wayback Machine. Retrieved 29 September 2013.
- ↑ Simeon J, Maguire J, Lawrence S (1981). Maprotiline effects in children with enuresis and behavioural disorders. Progress in Neuro-Psychopharmacology 5 ( 5–6), 495–8
- ↑ U.S. National Library of Medicine. Last Reviewed 1 Sept. 2010 Medline Plus entry for Maprotiline Archived 2016-07-05 at the Wayback Machine
- ↑ 13.0 13.1 13.2 13.3 13.4 13.5 13.6 13.7 Thomas L. Lemke; David A. Williams (24 January 2012). Foye's Principles of Medicinal Chemistry. Lippincott Williams & Wilkins. pp. 591–. ISBN 978-1-60913-345-0. Archived from the original on 2 January 2020. Retrieved 26 October 2021.
- ↑ 14.0 14.1 14.2 14.3 14.4 14.5 14.6 14.7 14.8 14.9 Yong Zhou (22 October 2013). Drugs in Psychiatric Practice. Elsevier. pp. 222–. ISBN 978-1-4831-9193-5.
- ↑ Roth, BL; Driscol, J. "PDSP Ki Database". Psychoactive Drug Screening Program (PDSP). University of North Carolina at Chapel Hill and the United States National Institute of Mental Health. Archived from the original on 26 January 2021. Retrieved 14 August 2017.
- ↑ 16.0 16.1 16.2 Tatsumi M, Groshan K, Blakely RD, Richelson E (1997). "Pharmacological profile of antidepressants and related compounds at human monoamine transporters". Eur. J. Pharmacol. 340 (2–3): 249–58. doi:10.1016/s0014-2999(97)01393-9. PMID 9537821.
- ↑ Heffernan GD, Coghlan RD, Manas ES, McDevitt RE, Li Y, Mahaney PE, Robichaud AJ, Huselton C, Alfinito P, Bray JA, Cosmi SA, Johnston GH, Kenney T, Koury E, Winneker RC, Deecher DC, Trybulski EJ (2009). "Dual acting norepinephrine reuptake inhibitors and 5-HT(2A) receptor antagonists: Identification, synthesis and activity of novel 4-aminoethyl-3-(phenylsulfonyl)-1H-indoles". Bioorg. Med. Chem. 17 (22): 7802–15. doi:10.1016/j.bmc.2009.09.023. PMID 19836247.
- ↑ 18.0 18.1 Pälvimäki EP, Roth BL, Majasuo H, Laakso A, Kuoppamäki M, Syvälahti E, Hietala J (1996). "Interactions of selective serotonin reuptake inhibitors with the serotonin 5-HT2c receptor". Psychopharmacology. 126 (3): 234–40. doi:10.1007/bf02246453. PMID 8876023. S2CID 24889381.
- ↑ Lucchelli A, Santagostino-Barbone MG, D'Agostino G, Masoero E, Tonini M (2000). "The interaction of antidepressant drugs with enteric 5-HT7 receptors". Naunyn Schmiedebergs Arch. Pharmacol. 362 (3): 284–9. doi:10.1007/s002100000295. PMID 10997731. S2CID 24189673.
- ↑ 20.0 20.1 20.2 20.3 20.4 Richelson E, Nelson A (1984). "Antagonism by antidepressants of neurotransmitter receptors of normal human brain in vitro". J. Pharmacol. Exp. Ther. 230 (1): 94–102. PMID 6086881.
- ↑ 21.0 21.1 21.2 21.3 21.4 21.5 von Coburg Y, Kottke T, Weizel L, Ligneau X, Stark H (2009). "Potential utility of histamine H3 receptor antagonist pharmacophore in antipsychotics". Bioorg. Med. Chem. Lett. 19 (2): 538–42. doi:10.1016/j.bmcl.2008.09.012. PMID 19091563.
- ↑ 22.0 22.1 22.2 Appl H, Holzammer T, Dove S, Haen E, Strasser A, Seifert R (2012). "Interactions of recombinant human histamine H1R, H2R, H3R, and H4R receptors with 34 antidepressants and antipsychotics". Naunyn Schmiedebergs Arch. Pharmacol. 385 (2): 145–70. doi:10.1007/s00210-011-0704-0. PMID 22033803. S2CID 14274150.
- ↑ Kanba S, Richelson E (1984). "Histamine H1 receptors in human brain labelled with [3H]doxepin". Brain Res. 304 (1): 1–7. doi:10.1016/0006-8993(84)90856-4. PMID 6146381. S2CID 45303586.
- ↑ El-Fakahany E, Richelson E (1983). "Antagonism by antidepressants of muscarinic acetylcholine receptors of human brain". Br. J. Pharmacol. 78 (1): 97–102. doi:10.1111/j.1476-5381.1983.tb17361.x. PMC 2044798. PMID 6297650.
- ↑ Peng, Wen-Huang; Kuan-Lin Lo; Yi-Hsuen Lee; Tai-Huang Hung; Ying-Chih Lin (2007). "Berberine produces antidepressant-like effects in the forced swim test and in the tail suspension test in mice". Life Sciences. 81 (11): 933–938. doi:10.1016/j.lfs.2007.08.003. PMID 17804020.
- ↑ Matthys A, Haegeman G, Van Craenenbroeck K, Vanhoenacker P (June 2011). "Role of the 5-HT7 receptor in the central nervous system: from current status to future perspectives". Mol. Neurobiol. 43 (3): 228–53. doi:10.1007/s12035-011-8175-3. PMID 21424680. S2CID 25515856.
- ↑ Alan F. Schatzberg; Charles B. Nemeroff (2009). The American Psychiatric Publishing Textbook of Psychopharmacology. American Psychiatric Pub. pp. 277–. ISBN 978-1-58562-309-9. Archived from the original on 2021-08-27. Retrieved 2021-10-26.
- ↑ Miyake K, Fukuchi H, Kitaura T, Kimura M, Kimura Y, Nakahara T (1991). Pharmacokinetics of maprotiline and its demethylated metabolite in serum and specific brain regions of rats after acute and chronic administration of maprotiline. J Pharm Sci.;80(12):1114-8.
- ↑ 29.0 29.1 Ruben Vardanyan; Victor Hruby (10 March 2006). Synthesis of Essential Drugs. Elsevier. pp. 110–. ISBN 978-0-08-046212-7.
- ↑ 30.0 30.1 30.2 Daniel Lednicer; Lester A. Mitscher (13 May 1980). The Organic Chemistry of Drug Synthesis. John Wiley & Sons. pp. 220–. ISBN 978-0-471-04392-8.
- ↑ 31.0 31.1 31.2 31.3 Andersen J, Kristensen AS, Bang-Andersen B, Strømgaard K (2009). "Recent advances in the understanding of the interaction of antidepressant drugs with serotonin and norepinephrine transporters". Chem. Commun. (25): 3677–92. doi:10.1039/b903035m. PMID 19557250.
- ↑ 32.0 32.1 Richard C. Dart (2004). Medical Toxicology. Lippincott Williams & Wilkins. pp. 836–. ISBN 978-0-7817-2845-4.
- ↑ I.K. Morton; Judith M. Hall (31 October 1999). Concise Dictionary of Pharmacological Agents: Properties and Synonyms. Springer Science & Business Media. pp. 171–. ISBN 978-0-7514-0499-9.
External links
| Identifiers: |
|
|---|
- Pages using duplicate arguments in template calls
- Webarchive template wayback links
- Articles with changed InChI identifier
- Chemical articles with unknown parameter in Infobox drug
- Chemical articles without CAS registry number
- Articles without EBI source
- Chemical pages without ChemSpiderID
- Chemical pages without DrugBank identifier
- Articles without KEGG source
- Articles without UNII source
- Drugs missing an ATC code
- Drugboxes which contain changes to verified fields
- Drugboxes which contain changes to watched fields
- Articles with hatnote templates targeting a nonexistent page
- Articles with changed ChemSpider identifier
- Alpha-1 blockers
- Amines
- Anthracenes
- Novartis brands
- Antihistamines
- H1 receptor antagonists
- Muscarinic antagonists
- Norepinephrine reuptake inhibitors
- Serotonin antagonists
- Tetracyclic antidepressants
- Tricyclic antidepressants
- RTT