Mirabegron
 | |
| Names | |
|---|---|
| Trade names | Myrbetriq, Betanis, Betmiga, others |
| Other names | YM-178 |
| |
| Clinical data | |
| Drug class | β3 adrenergic receptor agonist[1][2] |
| Main uses | Overactive bladder[1] |
| Side effects | High blood pressure, headaches, urinary tract infections[1] |
| Pregnancy category |
|
| Routes of use | By mouth (tablets) |
| Defined daily dose | 50 mg[3] |
| External links | |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a612038 |
| Legal | |
| License data |
|
| Legal status | |
| Pharmacokinetics | |
| Bioavailability | 29–35%[4] |
| Protein binding | 71%[4] |
| Metabolism | Liver via (direct) glucuronidation, amide hydrolysis, and minimal oxidative metabolism in vivo by CYP2D6 and CYP3A4. Some involvement of butylcholinesterase[4] |
| Elimination half-life | 50 hours[4] |
| Excretion | Urine (55%), faeces (34%)[4] |
| Chemical and physical data | |
| Formula | C21H24N4O2S |
| Molar mass | 396.51 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Mirabegron, sold under the brand name Myrbetriq among others, is a medication used to treat overactive bladder.[1] Its benefits are similar to antimuscarinic medication such as solifenacin or tolterodine.[5] In the United Kingdom it is less preferred to antimuscarinic medication such as oxybutynin.[2] It is taken by mouth.[1]
Common side effects include high blood pressure, headaches, and urinary tract infections.[1] Other significant side effects include urinary retention, irregular heart rate, and angioedema.[1][2] It works by activating the β3 adrenergic receptor in the bladder, resulting in its relaxation.[1][2]
Mirabegron was approved for medical use in the United States and in the European Union in 2012.[6][7][8] A month supply in the United Kingdom costs the NHS about £29 as of 2019.[2] In the United States the wholesale cost of this amount is about 369 USD.[9] In 2017, it was the 191st most commonly prescribed medication in the United States, with more than three million prescriptions.[10][11]
Medical uses

Its used is in the treatment of overactive bladder.[4][12][13] It works equally well to antimuscarinic medication such as solifenacin or tolterodine.[5][8] In the United Kingdom it is less preferred to these agents.[2]
Dosage
The defined daily dose is 50 mg by mouth.[3]
Side effects
Side effects by frequency:[4][12][13]
Very common (>10% incidence):
Common (1–10% incidence):
- Dry mouth
- Nasopharyngitis
- Urinary tract infection (UTI)
- Headache
- Influenza
- Constipation
- Dizziness
- Joint pain
- Cystitis
- Back pain
- Upper respiratory tract infection (URTI)
- Sinusitis
- Diarrhea
- High heart rate
- Fatigue
- Abdominal pain
- Neoplasms (cancers)
Rare (<1% incidence):
- Palpitations
- Blurred vision
- Glaucoma
- Indigestion
- Gastritis
- Abdominal distension
- Rhinitis
- Elevations in liver enzymes (GGTP, AST, ALT and LDH)
- Renal and urinary disorders (e.g., nephrolithiasis, bladder pain)
- Reproductive system disorders (e.g., vulvovaginal pruritus, vaginal infection)
- Skin and subcutaneous tissue disorders (e.g., urticaria, leukocytoclastic vasculitis, rash, pruritus, purpura, lip edema)
- Stevens–Johnson syndrome associated with increased serum ALT, AST and bilirubin
- Urinary retention
Pronunciation
The brand name Myrbetriq is pronounced /mɪərˈbɛtrɪk/ meer-BET-rik.
Society and culture
Cost
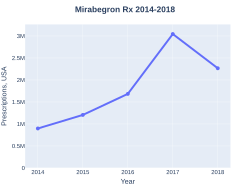
A month supply in the United Kingdom costs the NHS about £29 as of 2019.[2] In the United States the wholesale cost of this amount is about 369 USD.[9] In 2017, it was the 191st most commonly prescribed medication in the United States, with more than three million prescriptions.[10][11]
-
Mirabegron costs (US)
-
Mirabegron prescriptions (US)
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 1.6 1.7 "Mirabegron Monograph for Professionals". Drugs.com. American Society of Health-System Pharmacists. Archived from the original on 4 March 2021. Retrieved 18 March 2019.
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 2.6 British national formulary : BNF 76 (76 ed.). Pharmaceutical Press. 2018. p. 763. ISBN 9780857113382.
- ↑ 3.0 3.1 "WHOCC - ATC/DDD Index". www.whocc.no. Archived from the original on 16 April 2021. Retrieved 7 September 2020.
- ↑ 4.0 4.1 4.2 4.3 4.4 4.5 4.6 "mirabegron (Rx) - Myrbetriq". Medscape Reference. WebMD. Archived from the original on 11 February 2015. Retrieved 17 November 2013.
- ↑ 5.0 5.1 "[93] Are claims for newer drugs for overactive bladder warranted?". Therapeutics Initiative. 22 April 2015. Archived from the original on 2 April 2019. Retrieved 17 March 2019.
- ↑ "Drug Approval Package: Myrbetriq (mirabegron) Extended Release Tablets NDA #202611". U.S. Food and Drug Administration (FDA). 10 August 2012. Archived from the original on 8 April 2021. Retrieved 28 April 2020.
- ↑ Sacco E, Bientinesi R, Tienforti D, Racioppi M, Gulino G, D'Agostino D, et al. (April 2014). "Discovery history and clinical development of mirabegron for the treatment of overactive bladder and urinary incontinence". Expert Opinion on Drug Discovery. 9 (4): 433–48. doi:10.1517/17460441.2014.892923. PMID 24559030.
- ↑ 8.0 8.1 "Betmiga EPAR". European Medicines Agency. Archived from the original on 22 May 2020. Retrieved 28 April 2020. Text was copied from this source which is © European Medicines Agency. Reproduction is authorized provided the source is acknowledged.
- ↑ 9.0 9.1 "NADAC as of 2019-02-27". Centers for Medicare and Medicaid Services. Archived from the original on 6 March 2019. Retrieved 3 March 2019.
- ↑ 10.0 10.1 "The Top 300 of 2020". ClinCalc. Archived from the original on 12 February 2021. Retrieved 11 April 2020.
- ↑ 11.0 11.1 "Mirabegron - Drug Usage Statistics". ClinCalc. Archived from the original on 8 July 2020. Retrieved 11 April 2020.
- ↑ 12.0 12.1 "Myrbetriq (mirabegron) tablet, film coated, extended release [Astellas Pharma US, Inc.]". DailyMed. Astellas Pharma US, Inc. September 2012. Archived from the original on 28 November 2019. Retrieved 17 November 2013.
- ↑ 13.0 13.1 "Betmiga 25mg & 50mg prolonged-release tablets". electronic Medicines Compendium. Astellas Pharma Ltd. 22 February 2013. Archived from the original on 2 April 2015. Retrieved 17 November 2013.
External links
| External sites: | |
|---|---|
| Identifiers: |
|
- Sacco E, Bientinesi R (December 2012). "Mirabegron: a review of recent data and its prospects in the management of overactive bladder". Therapeutic Advances in Urology. 4 (6): 315–24. doi:10.1177/1756287212457114. PMC 3491758. PMID 23205058.
- Tyagi P, Tyagi V, Chancellor M (March 2011). "Mirabegron: a safety review". Expert Opin Drug Saf. 10 (2): 287–94. doi:10.1517/14740338.2011.542146. PMID 21142693.
- Pages using duplicate arguments in template calls
- Use dmy dates from April 2020
- Articles with invalid date parameter in template
- Drugs with non-standard legal status
- Chemical articles with unknown parameter in Infobox drug
- Chemical articles without CAS registry number
- Articles without EBI source
- Chemical pages without ChemSpiderID
- Chemical pages without DrugBank identifier
- Articles without KEGG source
- Articles without UNII source
- Drugs missing an ATC code
- Drug has EMA link
- Articles containing unverified chemical infoboxes
- Beta3-adrenergic agonists
- Thiazoles
- Astellas Pharma
- RTT