Glaucoma
| Glaucoma | |
|---|---|
 | |
| Acute angle closure glaucoma of the person's right eye (shown at left). Note the mid-sized pupil, which was non-reactive to light, and redness of the white part of the eye. | |
| Specialty | Ophthalmology |
| Symptoms | Vision loss, eye pain, mid-dilated pupil, redness of the eye, nausea[1][2] |
| Usual onset | Gradual, or sudden[2] |
| Risk factors | Increased pressure in the eye, family history, high blood pressure[1] |
| Diagnostic method | Dilated eye examination[1] |
| Differential diagnosis | Uveitis, trauma, keratitis, conjunctivitis[3] |
| Treatment | Medication, laser, surgery[1] |
| Frequency | 6–67 million[2][4] |
Glaucoma is a group of eye diseases which result in damage to the optic nerve and cause vision loss.[1] The most common type is open-angle glaucoma, in which the drainage angle for fluid within the eye remains open, with less common types including closed-angle glaucoma and normal-tension glaucoma.[1] Open-angle glaucoma develops slowly over time and there is no pain.[1] Peripheral vision may begin to decrease, followed by central vision, resulting in blindness if not treated.[1] Closed-angle glaucoma can present gradually or suddenly.[2] The sudden presentation may involve severe eye pain, blurred vision, mid-dilated pupil, redness of the eye, and nausea.[1][2] Vision loss from glaucoma, once it has occurred, is permanent.[1]
Risk factors for glaucoma include increased pressure in the eye, a family history of the condition, and high blood pressure.[1] For eye pressures, a value of greater than 21 mmHg or 2.8 kPa is often used, with higher pressures leading to a greater risk.[2][5] However, some may have high eye pressure for years and never develop damage.[2] Conversely, optic nerve damage may occur with normal pressure, known as normal-tension glaucoma.[6] The mechanism of open-angle glaucoma is believed to be slow exit of aqueous humor through the trabecular meshwork, while in closed-angle glaucoma the iris blocks the trabecular meshwork.[2] Diagnosis is by a dilated eye examination.[1] Often, the optic nerve shows an abnormal amount of cupping.[2]
If treated early, it is possible to slow or stop the progression of disease with medication, laser treatment, or surgery.[1][7] The goal of these treatments is to decrease eye pressure.[2] A number of different classes of glaucoma medication are available.[2] Laser treatments may be effective in both open-angle and closed-angle glaucoma.[2] A number of types of glaucoma surgeries may be used in people who do not respond sufficiently to other measures.[2] Treatment of closed-angle glaucoma is a medical emergency.[1]
About 6 to 67 million people have glaucoma globally.[2][4] The disease affects about 2 million people in the United States.[2] It occurs more commonly among older people.[1] Closed-angle glaucoma is more common in women.[2] Glaucoma has been called the "silent thief of sight", because the loss of vision usually occurs slowly over a long period of time.[8] Worldwide, glaucoma is the second-leading cause of blindness after cataracts.[2][9] Cataracts caused 51% of blindness in 2010, while glaucoma caused 8%.[10] The word "glaucoma" is from Ancient Greek glaukos, which means "shimmering".[11] In English, the word was used as early as 1587 but did not become commonly used until after 1850, when the development of the ophthalmoscope allowed people to see the optic nerve damage.[12]
Signs and symptoms

Open-angle glaucoma is painless and does not have acute attacks, thus the lack of clear symptoms make screening via regular eye check-ups important. The only signs are gradually progressive visual field loss, and optic nerve changes (increased cup-to-disc ratio on fundoscopic examination).
About 10% of people with closed angles present with acute angle closure characterized by sudden ocular pain, seeing halos around lights, red eye, very high intraocular pressure (>30 mmHg), nausea and vomiting, suddenly decreased vision, and a fixed, mid-dilated pupil. It is also associated with an oval pupil in some cases. Acute angle closure is an emergency.
Opaque specks may occur in the lens in glaucoma, known as glaukomflecken.[13]
Causes
Of the several causes for glaucoma, ocular hypertension (increased pressure within the eye) is the most important risk factor in most glaucomas, but in some populations, only 50% of people with primary open-angle glaucoma actually have elevated ocular pressure.[14] Ocular hypertension—an intraocular pressure above the traditional threshold of 21 mm Hg or even above 24 mm Hg—is not necessarily a pathological condition but it increases the risk of developing glaucoma. One study found a conversion rate of 18% within 5 years, meaning less than 1 in 5 people with an elevated intraocular pressure will develop glaucomatous visual field loss over that period of time.[15] It is a matter of debate whether every person with an elevated intraocular pressure should receive glaucoma therapy; currently most ophthalmologists favor treatment of people with additional risk factors.[16]
Open-angle glaucoma accounts for 90% of glaucoma cases in the United States. Closed-angle glaucoma accounts for less than 10% of glaucoma cases in the United States, but as many as half of glaucoma cases in other nations (particularly East Asian countries).
Dietary
No clear evidence indicates that vitamin deficiencies cause glaucoma. It follows, that vitamin supplementation is not a recommended treatment for glaucoma.[17] Caffeine increases intraocular pressure in those with glaucoma, but does not appear to affect normal individuals.[18]
Ethnicity
Many people of East Asian descent are prone to developing angle closure glaucoma due to shallower anterior chamber depths, with the majority of cases of glaucoma in this population consisting of some form of angle closure.[19] Higher rates of glaucoma have also been reported for Inuit populations, compared to White populations, in Canada and Greenland.[20]
Genetics
Positive family history is a risk factor for glaucoma. The relative risk of having primary open-angle glaucoma (P.O.A.G.) is increased about two- to four-fold for people who have a sibling with glaucoma.[21] Glaucoma, particularly primary open-angle glaucoma, is associated with mutations in several genes, including MYOC, ASB10, WDR36, NTF4, TBK1,[22] and RPGRIP1,[23] although most cases of glaucoma do not involve these genetic mutations. Normal-tension glaucoma, which comprises one-third of POAG, is also associated with genetic mutations (including OPA1 and OPTN genes).[24]
Various rare congenital/genetic eye malformations are associated with glaucoma. Occasionally, failure of the normal third-trimester gestational atrophy of the hyaloid canal and the tunica vasculosa lentis is associated with other anomalies. Angle closure-induced ocular hypertension and glaucomatous optic neuropathy may also occur with these anomalies,[25][26][27] and has been modelled in mice.[28]
Other
Other factors can cause glaucoma, known as "secondary glaucoma", including prolonged use of steroids (steroid-induced glaucoma); conditions that severely restrict blood flow to the eye, such as severe diabetic retinopathy and central retinal vein occlusion (neovascular glaucoma); ocular trauma (angle-recession glaucoma); and inflammation of the middle layer of the pigmented vascular eye structure (uveitis), known as uveitic glaucoma.
Pathophysiology

The underlying cause of open-angle glaucoma remains unclear. Several theories exist on its exact etiology. However, the major risk factor for most glaucomas and the focus of treatment is increased intraocular pressure. Intraocular pressure is a function of production of liquid aqueous humor by the ciliary processes of the eye, and its drainage through the trabecular meshwork. Aqueous humor flows from the ciliary processes into the posterior chamber, bounded posteriorly by the lens and the zonules of Zinn, and anteriorly by the iris. It then flows through the pupil of the iris into the anterior chamber, bounded posteriorly by the iris and anteriorly by the cornea. From here, the trabecular meshwork drains aqueous humor via the scleral venous sinus (Schlemm's canal) into scleral plexuses and general blood circulation.[29]
In open/wide-angle glaucoma, flow is reduced through the trabecular meshwork, due to the degeneration and obstruction of the trabecular meshwork, whose original function is to absorb the aqueous humor. Loss of aqueous humor absorption leads to increased resistance and thus a chronic, painless buildup of pressure in the eye.[30]
In close/narrow-angle, the iridocorneal angle is completely closed because of forward displacement of the final roll and root of the iris against the cornea, resulting in the inability of the aqueous fluid to flow from the posterior to the anterior chamber and then out of the trabecular network. This accumulation of aqueous humor causes an acute increase in pressure and pain.
The inconsistent relationship of glaucomatous optic neuropathy with increased intraocular pressure has provoked hypotheses and studies on anatomic structure, eye development, nerve compression trauma, optic nerve blood flow, excitatory neurotransmitter, trophic factor, retinal ganglion cell/axon degeneration, glial support cell, immune system, aging mechanisms of neuron loss, and severing of the nerve fibers at the scleral edge.[31][32][33][34][35][36][37][38][39][40][41]
Diagnosis

Screening for glaucoma is usually performed as part of a standard eye examination performed by optometrists and ophthalmologists. Testing for glaucoma should include measurements of the intraocular pressure via tonometry,[42] anterior chamber angle examination or gonioscopy, and examination of the optic nerve to look for any visible damage to it, or change in the cup-to-disc ratio and also rim appearance and vascular change. A formal visual field test should be performed. The retinal nerve fiber layer can be assessed with imaging techniques such as optical coherence tomography, scanning laser polarimetry, and/or scanning laser ophthalmoscopy (Heidelberg retinal tomogram).[43][44][45] Visual field loss is the most specific sign of the condition; however, it occurs later in the condition.[46]
Owing to the sensitivity of all methods of tonometry to corneal thickness, methods such as Goldmann tonometry should be augmented with pachymetry to measure the central corneal thickness (CCT). A thicker-than-average cornea can result in a pressure reading higher than the 'true' pressure whereas a thinner-than-average cornea can produce a pressure reading lower than the 'true' pressure.
Because pressure measurement error can be caused by more than just CCT (i.e., corneal hydration, elastic properties, etc.), it is impossible to 'adjust' pressure measurements based only on CCT measurements. The frequency doubling illusion can also be used to detect glaucoma with the use of a frequency doubling technology perimeter.[47]
Examination for glaucoma also could be assessed with more attention given to sex, race, history of drug use, refraction, inheritance and family history.[43]
| What the test examines | Eye drops used | Physical contact with the eye | Procedure | |
|---|---|---|---|---|
| Tonometry | Inner eye pressure | Maybe | Maybe | Eye drops may be used to numb the eye. The examiner then uses a tonometer to measure the inner pressure of the eye through pressure applied by a puff of warm air or a tiny tool. |
| Ophthalmoscopy (dilated eye examination) | Shape and color of the optic nerve | Yes | No | Eye drops are used to dilate the pupil. Using a small magnification device with a light on the end, the examiner can examine the magnified optic nerve. |
| Perimetry (visual field test) | Complete field of vision | No | No | The patient looks straight ahead and is asked to indicate when light passes the patient's peripheral field of vision. This allows the examiner to map the patient's field of vision. |
| Gonioscopy | Angle in the eye where the iris meets the cornea | Yes | Yes | Eye drops are used to numb the eye. A hand-held contact lens with a mirror is placed gently on the eye to allow the examiner to see the angle between the cornea and the iris. |
| Pachymetry | Thickness of the cornea | No | Yes | The examiner places a pachymeter gently on the front of the eye to measure its thickness. |
| Nerve fiber analysis | Thickness of the nerve fiber layer | Maybe | Maybe | Using one of several techniques,[clarification needed] the nerve fibers are examined. |
Glaucoma has been classified into specific types:[50]
Primary glaucoma and its variants
Primary glaucoma (H40.1-H40.2)
- Primary open-angle glaucoma, also known as chronic open-angle glaucoma, chronic simple glaucoma, glaucoma simplex
- High-tension glaucoma
- Low-tension glaucoma
- Primary angle closure glaucoma, also known as primary closed-angle glaucoma, narrow-angle glaucoma, pupil-block glaucoma, acute congestive glaucoma
- Acute angle closure glaucoma (aka AACG)[51]
- Chronic angle closure glaucoma
- Intermittent angle closure glaucoma
- Superimposed on chronic open-angle closure glaucoma ("combined mechanism" – uncommon)
Variants of primary glaucoma
- Pigmentary glaucoma
- Exfoliation glaucoma, also known as pseudoexfoliative glaucoma or glaucoma capsulare
- Primary juvenile glaucoma
Primary angle closure glaucoma is caused by contact between the iris and trabecular meshwork, which in turn obstructs outflow of the aqueous humor from the eye. This contact between iris and trabecular meshwork (TM) may gradually damage the function of the meshwork until it fails to keep pace with aqueous production, and the pressure rises. In over half of all cases, prolonged contact between iris and TM causes the formation of synechiae (effectively "scars").
These cause permanent obstruction of aqueous outflow. In some cases, pressure may rapidly build up in the eye, causing pain and redness (symptomatic, or so-called "acute" angle closure). In this situation, the vision may become blurred, and halos may be seen around bright lights. Accompanying symptoms may include a headache and vomiting.
Diagnosis is made from physical signs and symptoms: pupils mid-dilated and unresponsive to light, cornea edematous (cloudy), reduced vision, redness, and pain. However, the majority of cases are asymptomatic. Prior to the very severe loss of vision, these cases can only be identified by examination, generally by an eye care professional.
Once any symptoms have been controlled, the first line (and often definitive) treatment is laser iridotomy. This may be performed using either Nd:YAG or argon lasers, or in some cases by conventional incisional surgery. The goal of treatment is to reverse and prevent contact between the iris and trabecular meshwork. In early to moderately advanced cases, iridotomy is successful in opening the angle in around 75% of cases. In the other 25%, laser iridoplasty, medication (pilocarpine) or incisional surgery may be required.
Primary open-angle glaucoma is when optic nerve damage results in a progressive loss of the visual field.[52] This is associated with increased pressure in the eye. Not all people with primary open-angle glaucoma have eye pressure that is elevated beyond normal, but decreasing the eye pressure further has been shown to stop progression even in these cases.
The increased pressure is caused by trabecular meshwork blockage. Because the microscopic passageways are blocked, the pressure builds up in the eye and causes imperceptible very gradual vision loss. Peripheral vision is affected first, but eventually the entire vision will be lost if not treated.
Diagnosis is made by looking for cupping of the optic nerve. Prostaglandin agonists work by opening uveoscleral passageways. Beta-blockers, such as timolol, work by decreasing aqueous formation. Carbonic anhydrase inhibitors decrease bicarbonate formation from ciliary processes in the eye, thus decreasing the formation of aqueous humor. Parasympathetic analogs are drugs that work on the trabecular outflow by opening up the passageway and constricting the pupil. Alpha 2 agonists (brimonidine, apraclonidine) both decrease fluid production (via inhibition of AC) and increase drainage.
Developmental glaucoma
Developmental glaucoma (Q15.0)
- Primary congenital glaucoma
- Infantile glaucoma
- Glaucoma associated with hereditary or familial diseases
Secondary glaucoma
Secondary glaucoma (H40.3-H40.6)
- Inflammatory glaucoma
- Uveitis of all types
- Fuchs heterochromic iridocyclitis
- Phacogenic glaucoma
- Angle-closure glaucoma with mature cataract
- Phacoanaphylactic glaucoma secondary to rupture of lens capsule
- Phacolytic glaucoma due to phacotoxic meshwork blockage
- Subluxation of lens
- Glaucoma secondary to intraocular hemorrhage
- Hyphema
- Hemolytic glaucoma, also known as erythroclastic glaucoma
- Traumatic glaucoma
- Angle recession glaucoma: Traumatic recession on anterior chamber angle
- Postsurgical glaucoma
- Aphakic pupillary block
- Ciliary block glaucoma
- Neovascular glaucoma (see below for more details)
- Drug-induced glaucoma
- Corticosteroid induced glaucoma
- Alpha-chymotrypsin glaucoma. Postoperative ocular hypertension from use of alpha chymotrypsin.
- Glaucoma of miscellaneous origin
- Associated with intraocular tumors
- Associated with retinal detachments
- Secondary to severe chemical burns of the eye
- Associated with essential iris atrophy
- Toxic glaucoma
Neovascular glaucoma, an uncommon type of glaucoma, is difficult or nearly impossible to treat, and is often caused by proliferative diabetic retinopathy (PDR) or central retinal vein occlusion (CRVO). It may also be triggered by other conditions that result in ischemia of the retina or ciliary body. Individuals with poor blood flow to the eye are highly at risk for this condition.
Neovascular glaucoma results when new, abnormal vessels begin developing in the angle of the eye that begin blocking the drainage. Patients with such condition begin to rapidly lose their eyesight. Sometimes, the disease appears very rapidly, especially after cataract surgery procedures. A new treatment for this disease, as first reported by Kahook and colleagues, involves the use of a novel group of medications known as anti-VEGF agents. These injectable medications can lead to a dramatic decrease in new vessel formation and, if injected early enough in the disease process, may lead to normalization of intraocular pressure. Currently, there are no high-quality controlled trials demonstrating a beneficial effect of anti-VEGF treatments in lowering IOP in people with neovascular glaucoma.[53]
Toxic glaucoma is open-angle glaucoma with an unexplained significant rise of intraocular pressure following unknown pathogenesis. Intraocular pressure can sometimes reach 80 mmHg (11 kPa). It characteristically manifests as ciliary body inflammation and massive trabecular oedema that sometimes extends to Schlemm's canal. This condition is differentiated from malignant glaucoma by the presence of a deep and clear anterior chamber and a lack of aqueous misdirection. Also, the corneal appearance is not as hazy. A reduction in visual acuity can occur followed neuroretinal breakdown.
Associated factors include inflammation, drugs, trauma and intraocular surgery, including cataract surgery and vitrectomy procedures. Gede Pardianto (2005) reported on four patients who had toxic glaucoma. One of them underwent phacoemulsification with small particle nucleus drops. Some cases can be resolved with some medication, vitrectomy procedures or trabeculectomy. Valving procedures can give some relief, but further research is required.[54]
Absolute glaucoma
Absolute glaucoma (H44.5) is the end stage of all types of glaucoma. The eye has no vision, absence of pupillary light reflex and pupillary response, and has a stony appearance. Severe pain is present in the eye. The treatment of absolute glaucoma is a destructive procedure like cyclocryoapplication, cyclophotocoagulation, or injection of 99% alcohol.
Types
Glaucoma is an umbrella term for eye conditions which damage the optic nerve, and which can lead to a loss of vision.[55] The main cause of damage to the optic nerve is intraocular pressure (IOP), excessive fluid pressure within the eye, which can be due to various reasons including blockage of drainage ducts, and narrowing or closure of the angle between the iris and cornea.
The primary division in categorizing different types of glaucoma is open-angle and closed-angle (or angle-closure) glaucoma. In open angle glaucoma, the iris meets the cornea normally, allowing the fluid from inside the eye to drain, thus relieving the internal pressure. Where this angle is narrowed or closed, pressure increases over time, causing damage to the optic nerve, leading to blindness.
Primary open-angle glaucoma (also, primary glaucoma, chronic glaucoma) refers to slow clogging of the drainage canals resulting in increased eye pressure which causes progressive optic nerve damage. This manifests as a gradual loss of the visual field, starting with a loss of peripheral vision, but eventually the entire vision will be lost if not treated.[52] This is the most common type of glaucoma, accounting for 90% of cases in the United States, but fewer in Asian countries. Onset is slow and painless, and loss of vision is gradual and irreversible.
In narrow-angle glaucoma (also closed-angle glaucoma) the iris bows forward, narrowing the angle that drains the eye, increasing pressure within the eye. If untreated, it can lead to the medical emergency of angle-closure glaucoma.
In angle-closure glaucoma (also closed-angle glaucoma, primary angle-closure glaucoma, acute glaucoma) the iris bows forward and causes physical contact between the iris and trabecular meshwork, which blocks the outflow of aqueous humor from within the eye. This contact may gradually damage the draining function of the meshwork until it fails to keep pace with aqueous production, and the intraocular pressure rises. The onset of symptoms is sudden and causes pain and other symptoms that are noticeable; it is treated as a medical emergency. Unlike open-angle glaucoma, angle-closure glaucoma is a result of the angle between the iris and cornea closing. This tends to occur in the far-sighted, who have smaller-than-normal anterior chambers, making physical contact between the iris and trabecular meshwork more likely.
Normal-tension glaucoma (also NTG, low-tension glaucoma, normal-pressure glaucoma) is a condition where the optic nerve is damaged although intraocular pressure (IOP) is in the normal range (12-22mm Hg). Individuals with a family history of NTG, those of Japanese ancestry, those with a history of systemic heart disease, and those with Flammer syndrome are at a higher than average risk of developing NTG. The cause of NTG is unknown.
Secondary glaucoma refers to any case in which another disease, trauma, drug or procedure causes increased eye pressure, resulting in optic nerve damage and vision loss, and may be mild or severe. It can be due to an eye injury, inflammation, a tumor, or advanced cases of cataracts or diabetes. It can also be caused by certain drugs such as steroids. Treatment depends on whether it is open-angle or angle-closure glaucoma.
In pseudoexfoliation glaucoma (also, PEX, exfoliation glaucoma) the pressure is due to the accumulation of microscopic granular protein fibers, which can block normal drainage of the aqueous humor. PEX is prevalent in Scandinavia, primarily in those over 70, and more commonly in women.
Pigmentary glaucoma (also, pigmentary dispersion syndrome) is caused by pigment cells sloughing off from the back of the iris and floating around in the aqueous humor. Over time, these pigment cells can accumulate in the anterior chamber in such a way that it can begin to clog the trabecular meshwork. It is a rare condition that occurs mostly among Caucasians, mostly males in their mid-20s to 40s, and most are nearsighted.
Primary juvenile glaucoma is a neonate or juvenile abnormality where ocular hypertension is evident at birth or shortly thereafter and is caused by abnormalities in the anterior chamber angle development that blocks the outflow of the aqueous humor.
Uveitic glaucoma is due to uveitis, the swelling and inflammation of the uvea, the middle layer of the eye. The uvea provides most of the blood supply to the retina. Increased eye pressure in uveitis can result from the inflammation itself or from the steroids used to treat it.
Visual field defects

In glaucoma visual field defects result from damage to the retinal nerve fiber layer. Field defects are seen mainly in primary open angle glaucoma. Because of the unique anatomy of the RNFL, many noticeable patterns are seen in the visual field. Most of the early glaucomatous changes are seen within the central visual field,mainly in Bjerrum's area, 10-20° from fixation.[56]
Following are the common field defects in glaucoma:
- Generalized depression: Generalized depression is seen in early stages of glaucoma and many other conditions. Mild constriction of central and peripheral visual field due to isopter contraction comes under generalized depression. If all the isopters show similar depression to the same point, it is then called a contraction of visual field. Relative paracentral scotomas are the areas where smaller and dimmer targets are not visualized by the patient.[56] Larger and brighter targets can be seen. Small paracentral depressions, mainly superonasal are seen in normal tension glaucoma (NTG).[57] The generalized depression of the entire field may be seen in cataract also. [58]
- Baring of blind spot: Baring of blind spot means Exclusion of blind spot from the central field due to inward curve of the outer boundary of 30° central field [59]. It is only an early non-specific visual field change, without much diagnostic value in glaucoma. [59]
- Small wing-shaped paracentral scotoma: Small wing-shaped paracentral scotoma within Bjerrum’s area is the earliest clinically significant field defect seen in glaucoma. It may also be associated with nasal steps. Scotoma may be seen above or below blind spot.[59]
- Siedel’s sickle-shaped scotoma: Paracentral scotoma joins with the blind spot to form the Seidel sign.

- Arcuate or Bjerrum’s scotoma: It is formed at later stages of glaucoma by extension of Seidel’s scotoma in an area either above or below the fixation point to reach the horizontal line. Peripheral breakthrough may occur due to damage of nerve fibers. [59]
- Ring or Double arcuate scotoma: Two arcuate scotomas join together to form a Ring or Double arcuate scotoma. This defect is seen in advanced stages of glaucoma.
- Roenne’s central nasal step: It is created when two arcuate scotomas run in different arcs to form a right angled defect. This is also seen in advanced stages of glaucoma.
- Peripheral field defects: Peripheral field defects may occur in early or late stages of glaucoma. Roenne’s peripheral nasal steps occur due to contraction of peripheral isopter.[59]

- Tubular vision: Since macular fibers are the most resistant to glaucomatous damage, the central vision remains unaffected until end stages of glaucoma. Tubular vision or Tunnel vision is the loss of peripheral vision with retention of central vision, resulting in a constricted circular tunnel-like field of vision. It is seen in the end stages of glaucoma. Retinitis pigmentosa, is another disease that causes tubular vision.[60]
- Temporal island of vision: It is also seen in end stages of glaucoma. The temporal islands lie outside of the central 24 to 30° visual field,[61] so it may not be visible with standard central field measurements done in glaucoma.
Screening
The United States Preventive Services Task Force stated, as of 2013, that there was insufficient evidence to recommend for or against screening for glaucoma.[62] Therefore, there is no national screening program in the US. Screening, however, is recommended starting at age 40 by the American Academy of Ophthalmology.[2]
There is a glaucoma screening program in the UK. Those at risk are advised to have a dilated eye examination at least once a year.[63]
Treatment
The modern goals of glaucoma management are to avoid glaucomatous damage and nerve damage, and preserve visual field and total quality of life for patients, with minimal side-effects.[64][65] This requires appropriate diagnostic techniques and follow-up examinations, and judicious selection of treatments for the individual patient. Although intraocular pressure (IOP) is only one of the major risk factors for glaucoma, lowering it via various pharmaceuticals and/or surgical techniques is currently the mainstay of glaucoma treatment. A review of people with primary open-angle glaucoma and ocular hypertension concluded that medical IOP-lowering treatment slowed down the progression of visual field loss.[7]
Vascular flow and neurodegenerative theories of glaucomatous optic neuropathy have prompted studies on various neuroprotective therapeutic strategies, including nutritional compounds, some of which may be regarded by clinicians as safe for use now, while others are on trial.[citation needed]
Medication

Intraocular pressure can be lowered with medication, usually eye drops. Several classes of medications are used to treat glaucoma, with several medications in each class.
Each of these medicines may have local and systemic side effects. Adherence to medication protocol can be confusing and expensive; if side effects occur, the patient must be willing either to tolerate them or to communicate with the treating physician to improve the drug regimen. Initially, glaucoma drops may reasonably be started in either one or in both eyes.[66] Wiping the eye with an absorbent pad after the administration of eye drops may result in fewer adverse effects, like the growth of eyelashes and hyperpigmentation in the eyelid.[67]
Poor compliance with medications and follow-up visits is a major reason for vision loss in glaucoma patients. A 2003 study of patients in an HMO found half failed to fill their prescriptions the first time, and one-quarter failed to refill their prescriptions a second time.[68] Patient education and communication must be ongoing to sustain successful treatment plans for this lifelong disease with no early symptoms.
The possible neuroprotective effects of various topical and systemic medications are also being investigated.[17][69][70][71]
- Prostaglandin analogs, such as latanoprost, bimatoprost and travoprost, increase uveoscleral outflow of aqueous humor. Bimatoprost also increases trabecular outflow.
- Topical beta-adrenergic receptor antagonists, such as timolol, levobunolol, and betaxolol, decrease aqueous humor production by the epithelium of the ciliary body.
- Alpha2-adrenergic agonists, such as brimonidine and apraclonidine, work by a dual mechanism, decreasing aqueous humor production and increasing uveoscleral outflow.
- Less-selective alpha agonists, such as epinephrine, decrease aqueous humor production through vasoconstriction of ciliary body blood vessels, useful only in open-angle glaucoma. Epinephrine's mydriatic effect, however, renders it unsuitable for closed-angle glaucoma due to further narrowing of the uveoscleral outflow (i.e. further closure of trabecular meshwork, which is responsible for absorption of aqueous humor).
- Miotic agents (parasympathomimetics), such as pilocarpine, work by contraction of the ciliary muscle, opening the trabecular meshwork and allowing increased outflow of the aqueous humour. Echothiophate, an acetylcholinesterase inhibitor, is used in chronic glaucoma.
- Carbonic anhydrase inhibitors, such as dorzolamide, brinzolamide, and acetazolamide, lower secretion of aqueous humor by inhibiting carbonic anhydrase in the ciliary body.
Laser
Argon laser trabeculoplasty (ALT) may be used to treat open-angle glaucoma, but this is a temporary solution, not a cure. A 50-μm argon laser spot is aimed at the trabecular meshwork to stimulate the opening of the mesh to allow more outflow of aqueous fluid. Usually, half of the angle is treated at a time. Traditional laser trabeculoplasty uses a thermal argon laser in an argon laser trabeculoplasty procedure.
Nd:YAG laser peripheral iridotomy (LPI) may be used in patients susceptible to or affected by angle closure glaucoma or pigment dispersion syndrome. During laser iridotomy, laser energy is used to make a small, full-thickness opening in the iris to equalize the pressure between the front and back of the iris, thus correcting any abnormal bulging of the iris. In people with narrow angles, this can uncover the trabecular meshwork. In some cases of intermittent or short-term angle closure, this may lower the eye pressure. Laser iridotomy reduces the risk of developing an attack of acute angle closure. In most cases, it also reduces the risk of developing chronic angle closure or of adhesions of the iris to the trabecular meshwork.
Diode laser cycloablation lowers IOP by reducing aqueous secretion by destroying secretory ciliary epithelium.[43]
Surgery
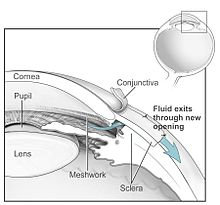
Both laser and conventional surgeries are performed to treat glaucoma. Surgery is the primary therapy for those with congenital glaucoma.[72] Generally, these operations are a temporary solution, as there is not yet a cure for glaucoma.
Canaloplasty
Canaloplasty is a nonpenetrating procedure using microcatheter technology. To perform a canaloplasty, an incision is made into the eye to gain access to the Schlemm's canal in a similar fashion to a viscocanalostomy. A microcatheter will circumnavigate the canal around the iris, enlarging the main drainage channel and its smaller collector channels through the injection of a sterile, gel-like material called viscoelastic. The catheter is then removed and a suture is placed within the canal and tightened.
By opening the canal, the pressure inside the eye may be relieved, although the reason is unclear, since the canal (of Schlemm) does not have any significant fluid resistance in glaucoma or healthy eyes. Long-term results are not available.[73][74]
Trabeculectomy
The most common conventional surgery performed for glaucoma is the trabeculectomy. Here, a partial thickness flap is made in the scleral wall of the eye, and a window opening is made under the flap to remove a portion of the trabecular meshwork. The scleral flap is then sutured loosely back in place to allow fluid to flow out of the eye through this opening, resulting in lowered intraocular pressure and the formation of a bleb or fluid bubble on the surface of the eye.
Scarring can occur around or over the flap opening, causing it to become less effective or lose effectiveness altogether. Traditionally, chemotherapeutic adjuvants, such as mitomycin C (MMC) or 5-fluorouracil (5-FU), are applied with soaked sponges on the wound bed to prevent filtering blebs from scarring by inhibiting fibroblast proliferation. Contemporary alternatives to prevent the scarring of the meshwork opening include the sole or combinative implementation of nonchemotherapeutic adjuvants such as the ologen collagen matrix, which has been clinically shown to increase the success rates of surgical treatment.[75][76][77][78]
Collagen matrix prevents scarring by randomizing and modulating fibroblast proliferation in addition to mechanically preventing wound contraction and adhesion.
Glaucoma drainage implants
The first glaucoma drainage implant was developed in 1966.[79] Since then, several types of implants have followed on from the original: the Baerveldt tube shunt, or the valved implants, such as the Ahmed glaucoma valve implant or the ExPress Mini Shunt and the later generation pressure ridge Molteno implants. These are indicated for glaucoma patients not responding to maximal medical therapy, with previous failed guarded filtering surgery (trabeculectomy). The flow tube is inserted into the anterior chamber of the eye, and the plate is implanted underneath the conjunctiva to allow a flow of aqueous fluid out of the eye into a chamber called a bleb.
- The first-generation Molteno and other nonvalved implants sometimes require the ligation of the tube until the bleb formed is mildly fibrosed and water-tight.[80] This is done to reduce postoperative hypotony—sudden drops in postoperative intraocular pressure.
- Valved implants, such as the Ahmed glaucoma valve, attempt to control postoperative hypotony by using a mechanical valve.
- Ab interno implants, such as the Xen Gel Stent, are transscleral implants by an ab interno procedure to channel aqueous humor into the non-dissected Tenon's space, creating a subconjunctival drainage area similar to a bleb.[81][82] The implants are transscleral and different from other ab interno implants that do not create a transscleral drainage, such as iStent, CyPass, or Hydrus.[83]
The ongoing scarring over the conjunctival dissipation segment of the shunt may become too thick for the aqueous humor to filter through. This may require preventive measures using antifibrotic medications, such as 5-fluorouracil or mitomycin-C (during the procedure), or other nonantifibrotic medication methods, such as collagen matrix implant,[84][85] or biodegradable spacer, or later on create a necessity for revision surgery with the sole or combinative use of donor patch grafts or collagen matrix implant.[86] And for glaucomatous painful blind eye and some cases of glaucoma, cyclocryotherapy for ciliary body ablation could be considered to be performed.[87]
Laser-assisted nonpenetrating deep sclerectomy
The most common surgical approach currently used for the treatment of glaucoma is trabeculectomy, in which the sclera is punctured to alleviate intraocular pressure.
Nonpenetrating deep sclerectomy (NPDS) surgery is a similar, but modified, procedure, in which instead of puncturing the scleral bed and trabecular meshwork under a scleral flap, a second deep scleral flap is created, excised, with further procedures of deroofing the Schlemm's canal, upon which, percolation of liquid from the inner eye is achieved and thus alleviating intraocular pressure, without penetrating the eye. NPDS is demonstrated to have significantly fewer side effects than trabeculectomy.[88] However, NPDS is performed manually and requires higher level of skills that may be assisted with instruments.[citation needed] In order to prevent wound adhesion after deep scleral excision and to maintain good filtering results, NPDS as with other non-penetrating procedures is sometimes performed with a variety of biocompatible spacers or devices, such as the Aquaflow collagen wick,[89] ologen Collagen Matrix,[77][90][91] or Xenoplast glaucoma implant.[92]
Laser-assisted NPDS is performed with the use of a CO2 laser system. The laser-based system is self-terminating once the required scleral thickness and adequate drainage of the intraocular fluid have been achieved. This self-regulation effect is achieved as the CO2 laser essentially stops ablating as soon as it comes in contact with the intraocular percolated liquid, which occurs as soon as the laser reaches the optimal residual intact layer thickness.
Prognosis
In open-angle glaucoma, the typical progression from normal vision to complete blindness takes about 25 years to 70 years without treatment, depending on the method of estimation used.[93] The intraocular pressure can also have an effect, with higher pressures reducing the time until blindness.[94]
Epidemiology

As of 2010, there were 44.7 million people in the world with open angle glaucoma.[96] The same year, there were 2.8 million people in the United States with open angle glaucoma.[96] By 2020, the prevalence is projected to increase to 58.6 million worldwide and 3.4 million in the United States.[96]
Both internationally and in the United States, glaucoma is the second-leading cause of blindness.[2] Globally, cataracts are a more common cause. Glaucoma is also the leading cause of blindness in African Americans, who have higher rates of primary open-angle glaucoma.[97][98] Bilateral vision loss can negatively affect mobility and interfere with driving.[99]
A meta-analysis published in 2009 found that people with primary open angle glaucoma do not have increased mortality rates, or increased risk of cardiovascular death.[100]
History
The association of elevated intraocular pressure (IOP) and glaucoma was first described by Englishman Richard Bannister in 1622: "...that the Eye be grown more solid and hard, then naturally it should be...".[101] Angle-closure glaucoma was treated with cataract extraction by John Collins Warren in Boston as early as 1806.[102] The invention of the ophthalmoscope by Hermann Helmholtz in 1851 enabled ophthalmologists for the first time to identify the pathological hallmark of glaucoma, the excavation of the optic nerve head due to retinal ganglion cell loss. The first reliable instrument to measure intraocular pressure was invented by Norwegian ophthalmologist Hjalmar August Schiøtz in 1905. About half a century later, Hans Goldmann in Berne, Switzerland, developed his applanation tonometer which still today - despite numerous new innovations in diagnostics - is considered the gold standard of determining this crucial pathogenic factor. In the late 20th century, further pathomechanisms beyond elevated IOP were discovered and became the subject of research like insufficient blood supply – often associated with low or irregular blood pressure – to the retina and optic nerve head.[103] The first drug to reduce IOP, pilocarpine, was introduced in the 1870s; other major innovations in pharmacological glaucoma therapy were the introduction of beta blocker eye drops in the 1970s and of prostaglandin analogues and topical (locally administered) carbonic anhydrase inhibitors in the mid-1990s.. Early surgical techniques like iridectomy and fistulating methods have recently been supplemented by less invasive procedures like small implants, a range of options now widely called MIGS (micro-invasive glaucoma surgery).
Etymology
The word "glaucoma" comes from the Ancient Greek γλαύκωμα,[104] a derivative of γλαυκóς,[105] which commonly described the color of eyes which were not dark (i.e. blue, green, light gray). Eyes described as γλαυκóς due to disease might have had a gray cataract in the Hippocratic era, or, in the early Common Era, the greenish pupillary hue sometimes seen in angle-closure glaucoma.[106][107]
Research
Rho kinase inhibitors
Rho kinase inhibitors, such as ripasudil, work by inhibition of the actin cytoskeleton, resulting in the morphological changes in the trabecular meshwork and increased aqueous outflow. More compounds in this class are being investigated in phase 2 and phase 3 trials.[108]
Neuroprotective agents
A 2013 Cochrane Systematic Review compared the effect of brimonidine and timolol in slowing the progression of open angle glaucoma in adult participants.[109] The results showed that participants assigned to brimonidine showed less visual field progression than those assigned to timolol, though the results were not significant, given the heavy loss-to-followup and limited evidence.[109] The mean intraocular pressures for both groups were similar. Participants in the brimonidine group had a higher occurrence of side effects caused by medication than participants in the timolol group.[109]
Cannabis
Studies in the 1970s reported that the use of cannabis may lower intraocular pressure.[110][111][112] In an effort to determine whether marijuana, or drugs derived from it, might be effective as a glaucoma treatment, the US National Eye Institute supported research studies from 1978 to 1984. These studies demonstrated some derivatives of marijuana lowered intraocular pressure when administered orally, intravenously, or by smoking, but not when topically applied to the eye.
In 2003, the American Academy of Ophthalmology released a position statement stating that cannabis was not more effective than prescription medications. Furthermore, no scientific evidence has been found that demonstrates increased benefits and/or diminished risks of cannabis use to treat glaucoma compared with the wide variety of pharmaceutical agents now available.[112][113]
In 2010 the American Glaucoma Society published a position paper discrediting the use of cannabis as a legitimate treatment for elevated intraocular pressure, for reasons including short duration of action and side effects that limit many activities of daily living.[114]
References
- ↑ 1.00 1.01 1.02 1.03 1.04 1.05 1.06 1.07 1.08 1.09 1.10 1.11 1.12 1.13 1.14 "Facts About Glaucoma". National Eye Institute. Archived from the original on 28 March 2016. Retrieved 29 March 2016.
- ↑ 2.00 2.01 2.02 2.03 2.04 2.05 2.06 2.07 2.08 2.09 2.10 2.11 2.12 2.13 2.14 2.15 2.16 2.17 2.18 Mantravadi, AV; Vadhar, N (September 2015). "Glaucoma". Primary Care. 42 (3): 437–49. doi:10.1016/j.pop.2015.05.008. ISSN 0095-4543. PMID 26319348.
- ↑ Ferri, Fred F. (2010). Ferri's differential diagnosis : a practical guide to the differential diagnosis of symptoms, signs, and clinical disorders (2nd ed.). Philadelphia, PA: Elsevier/Mosby. p. Chapter G. ISBN 978-0323076999.
- ↑ 4.0 4.1 GBD 2015 Disease and Injury Incidence and Prevalence, Collaborators. (8 October 2016). "Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015". Lancet. 388 (10053): 1545–1602. doi:10.1016/S0140-6736(16)31678-6. PMC 5055577. PMID 27733282.
- ↑ Rhee, Douglas J. (2012). Glaucoma (2 ed.). Philadelphia: Wolters Kluwer Health/Lippincott Williams & Wilkins. p. 180. ISBN 9781609133375. OCLC 744299538.
- ↑ Mi, Xue-Song; Yuan, Ti-Fei; So, Kwok-Fai (16 September 2014). "The current research status of normal tension glaucoma". Clinical Interventions in Aging. 9: 1563–71. doi:10.2147/CIA.S67263. PMC 4172068. PMID 25258525.
- ↑ 7.0 7.1 Vass, C.; Hirn, C.; Sycha, T.; Findl, O.; Bauer, P.; Schmetterer, L. (17 October 2007). "Medical interventions for primary open angle glaucoma and ocular hypertension". The Cochrane Database of Systematic Reviews (4): CD003167. doi:10.1002/14651858.CD003167.pub3. ISSN 1469-493X. PMC 6768994. PMID 17943780.
- ↑ "Glaucoma: The 'silent thief' begins to tell its secrets" (Press release). National Eye Institute. 21 January 2014. Archived from the original on 23 July 2015.
- ↑ Resnikoff, Serge; Pascolini, Donatella; Etya'Ale, Daniel; Kocur, Ivo; Pararajasegaram, Ramachandra; Pokharel, Gopal P.; Mariotti, Silvio P. (2004). "Global data on visual impairment in the year 2002". Bulletin of the World Health Organization. 82 (11): 844–51. doi:10.1590/S0042-96862004001100009 (inactive 7 June 2020). PMC 2623053. PMID 15640920. Archived from the original on 12 December 2013.
{{cite journal}}: CS1 maint: DOI inactive as of June 2020 (link) - ↑ GLOBAL DATA ON VISUAL IMPAIRMENTS 2010 (PDF). World Health Organization. 2010. p. 3. Archived (PDF) from the original on 10 June 2020. Retrieved 24 July 2020.
- ↑ "Greek Dictionary Headword Search Results". www.perseus.tufts.edu. Archived from the original on 27 April 2021. Retrieved 31 March 2020.
- ↑ Leffler CT, Schwartz SG, Stackhouse R, Davenport B, Spetzler K (2013). "Evolution and impact of eye and vision terms in written English". JAMA Ophthalmology. 131 (12): 1625–31. doi:10.1001/jamaophthalmol.2013.917. PMID 24337558. Archived from the original on 23 December 2014.
- ↑ Friedman, Neil J.; Kaiser, Peter K.; II, Roberto Pineda (2014). The Massachusetts Eye and Ear Infirmary Illustrated Manual of Ophthalmology E-Book. Elsevier Health Sciences. p. 234. ISBN 9780323225274. Archived from the original on 28 August 2021. Retrieved 24 July 2020.
- ↑ Sommer A, Tielsch JM, Katz J, et al. (August 1991). "Relationship between intraocular pressure and primary open angle glaucoma among white and black Americans. The Baltimore Eye Survey". Archives of Ophthalmology. 109 (8): 1090–95. doi:10.1001/archopht.1991.01080080050026. PMID 1867550.
- ↑ Kelly, Stephen R; Khawaja, Anthony P; Bryan, Susan R; Azuara-Blanco, Augusto; Sparrow, John M; Crabb, David P (25 March 2020). "Progression from ocular hypertension to visual field loss in the English hospital eye service". British Journal of Ophthalmology: bjophthalmol-2019-315052. doi:10.1136/bjophthalmol-2019-315052. PMID 32217541.
- ↑ Gordon, MO; Kass, MA (May 2018). "What We Have Learned From the Ocular Hypertension Treatment Study". American Journal of Ophthalmology. 189: xxiv–xxvii. doi:10.1016/j.ajo.2018.02.016. PMC 5915899. PMID 29501371.
- ↑ 17.0 17.1 Rhee DJ, Katz LJ, Spaeth GL, Myers JS (2001). "Complementary and alternative medicine for glaucoma". Survey of Ophthalmology. 46 (1): 43–55. doi:10.1016/S0039-6257(01)00233-8. PMID 11525790.
- ↑ Li, M; Wang, M; Guo, W; Wang, J; Sun, X (March 2011). "The effect of caffeine on intraocular pressure: a systematic review and meta-analysis". Graefe's Archive for Clinical and Experimental Ophthalmology = Albrecht von Graefes Archiv für Klinische und Experimentelle Ophthalmologie. 249 (3): 435–42. doi:10.1007/s00417-010-1455-1. ISSN 1435-702X. PMID 20706731.
- ↑ Wang N, Wu H, Fan Z (November 2002). "Primary angle closure glaucoma in Chinese and Western populations". Chinese Medical Journal. 115 (11): 1706–15. PMID 12609093. Archived from the original on 21 December 2016.
- ↑ Friedman D, Vedula SS (2006). "Lens extraction for chronic angle-closure glaucoma". Cochrane Database of Systematic Reviews. 3 (3): CD005555. doi:10.1002/14651858.CD005555.pub2. PMC 4438535. PMID 16856103.
- ↑ Myron Yanoff; Jay S. Duker (2009). Ophthalmology (3rd ed.). Mosby Elsevier. p. 1096. ISBN 9780323043328.
- ↑ Online Mendelian Inheritance in Man (OMIM): GLAUCOMA, PRIMARY OPEN ANGLE; POAG - 137760
- ↑ Fernández-Martínez, Lorena; Letteboer, Stef; Mardin, Christian Y.; Weisschuh, Nicole; Gramer, Eugen; Weber, Bernhard Hf; Rautenstrauss, Bernd; Ferreira, Paulo A.; Kruse, Friedrich E. (April 2011). "Evidence for RPGRIP1 gene as risk factor for primary open angle glaucoma". European Journal of Human Genetics. 19 (4): 445–451. doi:10.1038/ejhg.2010.217. ISSN 1476-5438. PMC 3060327. PMID 21224891.
- ↑ Online Mendelian Inheritance in Man (OMIM): GLAUCOMA, NORMAL TENSION, SUSCEPTIBILITY TO - 606657
- ↑ Pardianto G, et al. (2005). "Aqueous Flow and the Glaucoma". Mimbar Ilmiah Oftalmologi Indonesia. 2: 12–15.
- ↑ Chaum E, et al. "A 5-year-old girl who failed her school vision screening. Case presentation of Persistent fetal vasculature (PFV), also called persistent hyperplastic primary vitreous (PHPV)". Digital Journal of Ophthalmology. Archived from the original on 11 January 2009.
- ↑ Hunt A, Rowe N, Lam A, Martin F (July 2005). "Outcomes in persistent hyperplastic primary vitreous". Br J Ophthalmol. 89 (7): 859–63. doi:10.1136/bjo.2004.053595. PMC 1772745. PMID 15965167.
- ↑ Chang B, Smith RS, Peters M, et al. (2001). "Haploinsufficient Bmp4 ocular phenotypes include anterior segment dysgenesis with elevated intraocular pressure". BMC Genet. 2: 18. doi:10.1186/1471-2156-2-18. PMC 59999. PMID 11722794.
- ↑ Alguire P (1990). "The Eye Chapter 118 Tonometry>Basic Science". In Walker HK, Hall WD, Hurst JW (eds.). Clinical methods: the history, physical, and laboratory examinations (3rd ed.). London: Butterworths. ISBN 978-0-409-90077-4. Archived from the original on 22 July 2020. Retrieved 24 July 2020.
- ↑ Mozaffarieh M, Grieshaber MC, Flammer J (2008). "Oxygen and blood flow: players in the pathogenesis of glaucoma". Mol. Vis. 14: 224–33. PMC 2267728. PMID 18334938. Archived from the original on 9 June 2008.
- ↑ Osborne NN, Wood JP, Chidlow G, Bae JH, Melena J, Nash MS (August 1999). "Ganglion cell death in glaucoma: what do we really know?". Br J Ophthalmol. 83 (8): 980–6. doi:10.1136/bjo.83.8.980. PMC 1723166. PMID 10413706.
- ↑ Levin LA, Peeples P (February 2008). "History of neuroprotection and rationale as a therapy for glaucoma". Am J Manag Care. 14 (1 Suppl): S11–4. PMID 18284310. Archived from the original on 7 April 2012.
- ↑ Varma R, Peeples P, Walt JG, Bramley TJ (February 2008). "Disease progression and the need for neuroprotection in glaucoma management". Am J Manag Care. 14 (1 Suppl): S15–9. PMID 18284311. Archived from the original on 7 April 2012.
- ↑ Hernández M, Urcola JH, Vecino E (May 2008). "Retinal ganglion cell neuroprotection in a rat model of glaucoma following brimonidine, latanoprost or combined treatments". Exp. Eye Res. 86 (5): 798–806. doi:10.1016/j.exer.2008.02.008. PMID 18394603.
- ↑ Cantor LB (December 2006). "Brimonidine in the treatment of glaucoma and ocular hypertension". Ther Clin Risk Manag. 2 (4): 337–46. doi:10.2147/tcrm.2006.2.4.337. PMC 1936355. PMID 18360646.
- ↑ Schwartz M (June 2007). "Modulating the immune system: a vaccine for glaucoma?". Can J Ophthalmol. 42 (3): 439–41. doi:10.3129/I07-050. PMID 17508041.
- ↑ Morrison JC (2006). "Integrins in the optic nerve head: Potential roles in glaucomatous optic neuropathy (an American Ophthalmological Society thesis)". Trans Am Ophthalmol Soc. 104: 453–77. PMC 1809896. PMID 17471356.
- ↑ Knox DL, Eagle RC, Green WR (March 2007). "Optic nerve hydropic axonal degeneration and blocked retrograde axoplasmic transport: histopathologic features in human high-pressure secondary glaucoma". Arch. Ophthalmol. 125 (3): 347–53. doi:10.1001/archopht.125.3.347. PMID 17353405.
- ↑ Tezel G, Luo C, Yang X (March 2007). "Accelerated Aging in Glaucoma: Immunohistochemical Assessment of Advanced Glycation End Products in the Human Retina and Optic Nerve Head". Invest. Ophthalmol. Vis. Sci. 48 (3): 1201–11. doi:10.1167/iovs.06-0737. PMC 2492883. PMID 17325164.
- ↑ Berry FB, Mirzayans F, Walter MA (April 2006). "Regulation of FOXC1 stability and transcriptional activity by an epidermal growth factor-activated mitogen-activated protein kinase signaling cascade". J Biol Chem. 281 (15): 10098–104. doi:10.1074/jbc.M513629200. PMID 16492674.
- ↑ "Issue on neuroprotection". Can. J. Ophthalmol. 42 (3). June 2007. ISSN 1715-3360. Archived from the original on 12 May 2007.
- ↑ Farandos, NM; Yetisen, AK; Monteiro, MJ; Lowe, CR; Yun, SH (November 2014). "Contact Lens Sensors in Ocular Diagnostics". Advanced Healthcare Materials. 4 (6): 792–810. doi:10.1002/adhm.201400504. PMID 25400274.
- ↑ 43.0 43.1 43.2 Pardianto G et al. Some difficulties on Glaucoma. Mimbar Ilmiah Oftalmologi Indonesia. 2006;3: 49–52.
- ↑ Thomas R, Parikh RS (September 2006). "How to assess a patient for glaucoma". Community Eye Health. 19 (59): 36–7. PMC 1705629. PMID 17491713.
- ↑ Michelessi M, Lucenteforte E, Oddone F, Brazzelli M, Parravano M, Franchi S, Ng SM, Virgili G (2015). "Optic nerve head and fibre layer imaging for diagnosing glaucoma". Cochrane Database Syst Rev. 11 (11): CD008803. doi:10.1002/14651858.CD008803.pub2. PMC 4732281. PMID 26618332.
- ↑ Thylefors, B; Négrel, AD (1994). "The global impact of glaucoma". Bulletin of the World Health Organization. 72 (3): 323–6. PMC 2486713. PMID 8062393.
- ↑ Johnson, Chris A. The use of a visual illusion to detect glaucoma. In Visual Perception: The Influence of H. W. Leibowitz, eds. Andre, J., Owens, D. A., and Harvey, Jr., L. O. (2003); 45–56. Washington, D.C.: The American Psychological Association.
- ↑ Foundation, G. R. (n.d.). "Five common Glaucoma Tests". Glaucoma.org. Archived from the original on 8 September 2017. Retrieved 20 February 2014.
- ↑ "Nerve Fiber Analysis". Glaucoma Associates of Texas. White Rabbit Communications, Inc. 2010. Archived from the original on 26 March 2013. Retrieved 9 December 2012.
- ↑ Paton D, Craig JA; Craig (1976). "Glaucomas. Diagnosis and management". Clin Symp. 28 (2): 1–47. PMID 1053095.
- ↑ Logan, Carolynn M.; Rice, M. Katherine (1987). Logan's Medical and Scientific Abbreviations. Philadelphia: J. B. Lippincott Company. p. 3. ISBN 978-0-397-54589-6.
- ↑ 52.0 52.1 "Primary Open-Angle Glaucoma: Glaucoma: Merck Manual Professional". Merck.com. Archived from the original on 28 November 2010. Retrieved 24 January 2011.
- ↑ Simha A, Aziz K, Braganza A, Abraham L, Samuel P, Lindsley KB (2020). "Anti-vascular endothelial growth factor for neovascular glaucoma". Cochrane Database Syst Rev. 2 (2): CD007920. doi:10.1002/14651858.CD007920.pub3. PMC 7003996. PMID 32027392.
- ↑ Pardianto G, Difficulties on glaucoma in Mimbar Ilmiah Oftalmologi Indonesia.2006;3: 48–9.[verification needed]
- ↑ Arthur J. Sit, MD (23 April 2006). "Many types of glaucoma, one kind of damage to optic nerve". Chicago Tribune. Archived from the original on 6 October 2012. Retrieved 18 August 2015.
Glaucoma is a broad term for a number of different conditions that damage the optic nerve, the 'cable' that carries visual information from the eye to the brain, thereby causing changes in vision.
- ↑ 56.0 56.1 "Glaucoma". Parsons' diseases of the eye (22 ed.). Elsevier. 15 July 2015. pp. 288–295. ISBN 978-81-312-3818-9.
- ↑ John F, Salmon. "Glaucoma". Kanski's Clinical ophthalmology (9 ed.). Elsevier. pp. 362–365.
- ↑ Joy N. Carroll; Chris A. Johnson (22 August 2013). "Visual Field Testing: From One Medical Student to Another". Archived from the original on 4 August 2020. Retrieved 24 July 2020.
- ↑ 59.0 59.1 59.2 59.3 59.4 Khurana (31 August 2015). "Glaucoma". Comprehensive ophthalmology (6th ed.). Jaypee, The Health Sciences Publisher. pp. 223–224. ISBN 978-93-5152-657-5.
- ↑ "Retinitis pigmentosa". Genetics Home Reference. Archived from the original on 14 August 2020. Retrieved 24 July 2020.
- ↑ Themes, U. F. O. (11 July 2016). "Visual Fields in Glaucoma". Ento Key. Archived from the original on 9 August 2020. Retrieved 24 July 2020.
- ↑ Moyer, Virginia A. (9 July 2013). "Screening for Glaucoma: U.S. Preventive Services Task Force Recommendation Statement". Annals of Internal Medicine. 159 (7): I-28. doi:10.7326/0003-4819-159-6-201309170-00685. PMID 23836133.
- ↑ "Glaucoma – National Institutes of Health". Nihseniorhealth.gov. Archived from the original on 25 December 2010. Retrieved 24 January 2011.
- ↑ Noecker RJ (June 2006). "The management of glaucoma and intraocular hypertension: current approaches and recent advances". Ther Clin Risk Manag. 2 (2): 193–206. doi:10.2147/tcrm.2006.2.2.193. PMC 1661659. PMID 18360593.
- ↑ Parikh RS, Parikh SR, Navin S, Arun E, Thomas R (1 May 2008). "Practical approach to medical management of glaucoma". Indian J Ophthalmol. 56 (3): 223–30. doi:10.4103/0301-4738.40362. PMC 2636120. PMID 18417824.
- ↑ Leffler CT, Amini L (2007). "Interpretation of uniocular and binocular trials of glaucoma medications: an observational case series". BMC Ophthalmol. 7: 17. doi:10.1186/1471-2415-7-17. PMC 2093925. PMID 17916260.
- ↑ Xu L, Wang X, Wu M (2017). "Topical medication instillation techniques for glaucoma". Cochrane Database Syst Rev. 2: CD010520. doi:10.1002/14651858.CD010520.pub2. PMC 5419432. PMID 28218404.
- ↑ Jaret, Peter. "A New Understanding of Glaucoma". The New York Times. NYTimes.com. Archived from the original on 10 March 2014. Retrieved 20 February 2014.
- ↑ Ritch R (June 2007). "Natural compounds: evidence for a protective role in eye disease". Can. J. Ophthalmol. 42 (3): 425–38. doi:10.3129/I07-044. PMID 17508040.
- ↑ Tsai JC, Song BJ, Wu L, Forbes M (September 2007). "Erythropoietin: a candidate neuroprotective agent in the treatment of glaucoma". J Glaucoma. 16 (6): 567–71. doi:10.1097/IJG.0b013e318156a556. PMID 17873720.
- ↑ Mozaffarieh M, Flammer J (November 2007). "Is there more to glaucoma treatment than lowering IOP?". Surv Ophthalmol. 52 (Suppl 2): S174–79. doi:10.1016/j.survophthal.2007.08.013. PMID 17998043.
- ↑ Online Mendelian Inheritance in Man (OMIM): Glaucoma, Congenital: GLC3 Buphthalmos - 231300
- ↑ Shingleton B, Tetz M, Korber N (March 2008). "Circumferential viscodilation and tensioning of Schlemm's canal (canaloplasty) with temporal clear corneal phacoemulsification cataract surgery for open-angle glaucoma and visually significant cataract: one-year results". J Cataract Refract Surg. 34 (3): 433–40. doi:10.1016/j.jcrs.2007.11.029. PMID 18299068.
- ↑ Lewis RA, von Wolff K, Tetz M, et al. (July 2007). "Canaloplasty: circumferential viscodilation and tensioning of Schlemm's canal using a flexible microcatheter for the treatment of open-angle glaucoma in adults: interim clinical study analysis". J Cataract Refract Surg. 33 (7): 1217–26. doi:10.1016/j.jcrs.2007.03.051. PMID 17586378.
- ↑ Dada T, Sharma R, Sinha G, Angmo D, Temkar S (2016). "Cyclodialysis-enhanced trabeculectomy with triple Ologen implantation". Eur J Ophthalmol. 26 (1): 95–7. doi:10.5301/ejo.5000633. PMID 26044372.
- ↑ Yuan, F; Li, L; Chen; Yan; Wang (2015). "Biodegradable 3D-Porous Collagen Matrix (Ologen) Compared with Mitomycin C for Treatment of Primary Open-Angle Glaucoma: Results at 5 Years". Journal of Ophthalmology. 2015 (637537): 1–7. doi:10.1155/2015/637537. PMC 4452460. PMID 26078875.
- ↑ 77.0 77.1 Dada, Tanuj; Amit S; Saptorshi M; Meenakshi G (May 2013). "Combined Subconjunctival and Subscleral ologen Implant Insertion in Trabeculectomy". Eye. 27 (7): 889. doi:10.1038/eye.2013.76. PMC 3709396. PMID 23640614.
- ↑ Cillino, S; Casuccio A; Di Pace F; Cagini C; Ferraro LL (March 2016). "Biodegradable collagen matrix implant versus mitomycin-C in trabeculectomy: five-year follow-up". BMC Ophthalmol. 16 (24): 24. doi:10.1186/s12886-016-0198-0. PMC 4779569. PMID 26946419.
- ↑ "Eyelights Newsletter: About Glaucoma New Zealand" (PDF). Glaucoma.org. Archived (PDF) from the original on 13 January 2015. Retrieved 20 February 2014.
- ↑ Molteno AC, Polkinghorne PJ, Bowbyes JA (November 1986). "The vicryl tie technique for inserting a draining implant in the treatment of secondary glaucoma". Aust N Z J Ophthalmol. 14 (4): 343–54. doi:10.1111/j.1442-9071.1986.tb00470.x. PMID 3814422.
- ↑ Lewis RA (August 2014). "Ab interno approach to the subconjunctival space using a collagen glaucoma stent". J Cataract Refract Surg. 40 (8): 1301–6. doi:10.1016/j.jcrs.2014.01.032. PMID 24943904.
- ↑ "Xen Gel Stent". AqueSys. AqueSys. Archived from the original on 29 June 2015. Retrieved 27 June 2015.
- ↑ "Advances in Glaucoma Filtration Surgery". Glaucoma Today. Archived from the original on 29 June 2015. Retrieved 27 June 2015.
- ↑ Rosentreter, Andre; Andre M. Schild; Sven Dinslage; Thomas S. Dietlein (January 2011). "Biodegradable implant for tissue repair after glaucoma drainage device surgery". J Glaucoma. 21 (2): 76–8. doi:10.1097/IJG.0b013e3182027ab0. PMID 21278584.
- ↑ Rosentreter, Andre; Anne C. Mellein; Walter W. Konen; Thomas S. Dietlein (September 2010). "Capsule excision and ologenTM implantation for revision after glaucoma drainage device surgery". Graefes Arch Clin Exp Ophthalmol. 248 (9): 1319–24. doi:10.1007/s00417-010-1385-y. PMID 20405139.
- ↑ Rosentreter, A; Mellein AC; Konen WW; Dietlein TS (2010). "Capsule excision and ologenTM implantation for revision after glaucoma drainage device surgery". Graefes Arch Clin Exp Ophthalmol. 248 (9): 1319–24. doi:10.1007/s00417-010-1385-y. PMID 20405139.
- ↑ Pardianto G, et al. (2006). "Some difficulties on Glaucoma". Mimbar Ilmiah Oftalmologi Indonesia. 3: 49–50.
- ↑ Chiselita, D (2001). "Non-penetrating deep sclerectomy versus trabeculectomy in primary open-angle glaucoma surgery". Eye. 15 (Pt 2): 197–201. doi:10.1038/eye.2001.60. PMID 11339590.
- ↑ Iqbal "Ike" K. Ahmed (1 September 2005). "Making the Case for Nonpenetrating Surgery". Review of Ophthamology. 12 (9). Archived from the original on 11 October 2007.
- ↑ Aptel, F; Dumas S; Denis P (2009). "Ultrasound biomicroscopy and optical coherence tomography imaging of filtering blebs after deep sclerectomy with new collagen implant". Eur J Ophthalmol. 19 (2): 223–30. doi:10.1177/112067210901900208. PMID 19253238.
- ↑ Matthew, SJ; Sarkisian S; Nathan B; James MR (2012). "Initial experience using a collagen matrix implant (ologen) as a wound modulator with canaloplasty: 12 month results". Ft. Lauderdale: ARVO Congress. Archived from the original on 9 May 2020. Retrieved 24 July 2020.
- ↑ Anisimova SY, Anisimova SI, Larionov EV (2012). "Biological drainage – Xenoplast in glaucoma surgery (experimental and 10-year of clinical follow-up)" (PDF). Copenhagen: EGS Congress. Archived (PDF) from the original on 17 October 2013.
- ↑ Heijl, Anders; Bengtsson, Boel; Hyman, Leslie; Leske, M. Cristina (December 2009). "Natural History of Open-Angle Glaucoma". Ophthalmology (Submitted manuscript). 116 (12): 2271–76. doi:10.1016/j.ophtha.2009.06.042. PMID 19854514. Archived from the original on 28 August 2021. Retrieved 24 July 2020.
- ↑ "Glaucoma". Coopereyecare.com. 25 July 2013. Archived from the original on 13 December 2013. Retrieved 20 February 2014.
- ↑ "Death and DALY estimates for 2004 by cause for WHO Member States" (xls). World Health Organization. 2004. Archived from the original on 27 January 2012.
- ↑ 96.0 96.1 96.2 Quigley, H A; Broman, AT (March 2006). "The number of people with glaucoma worldwide in 2010 and 2020". British Journal of Ophthalmology. 90 (3): 262–67. doi:10.1136/bjo.2005.081224. PMC 1856963. PMID 16488940.
- ↑ Sommer, Alfred; Tielsch, James M.; Katz, Joanne; Quigley, Harry A.; Gottsch, John D.; Javitt, Jonathan C.; Martone, James F.; Royall, Richard M.; Witt, Kathe A.; Ezrine, Sandi (14 November 1991). "Racial Differences in the Cause-Specific Prevalence of Blindness in East Baltimore". New England Journal of Medicine. 325 (20): 1412–17. doi:10.1056/NEJM199111143252004. PMID 1922252.
- ↑ "Glaucoma and Marijuana use". National Eye Institute. 21 June 2005. Archived from the original on 27 December 2009.
- ↑ Ramulu, Pradeep (March 2009). "Glaucoma and disability: which tasks are affected, and at what stage of disease?". Current Opinion in Ophthalmology. 20 (2): 92–98. doi:10.1097/ICU.0b013e32832401a9. PMC 2692230. PMID 19240541.
- ↑ Akbari, M.; Akbari, S.; Pasquale, L. R. (February 2009). "The Association of Primary Open-angle Glaucoma With Mortality: A Meta-analysis of Observational Studies". Archives of Ophthalmology. 127 (2): 204–10. doi:10.1001/archophthalmol.2008.571. PMID 19204241.
- ↑ Richard Bannister: Treatise of One Hundred and Thirteen Diseases of the Eyes and Eyelids. London 1622
- ↑ Leffler CT, et al. (2017). "Ophthalmology in North America: Early Stories (1491-1801)". Ophthalmology and Eye Diseases. 9: 1–51. doi:10.1177/1179172117721902. PMC 5533269. PMID 28804247. Archived from the original on 3 October 2017. Retrieved 24 July 2020.
- ↑ Daniel Albert and Diane Edwards: The History of Ophthalmologist. Cambridge, Mass. 1996
- ↑ Harper, Douglas. "glaucoma". Online Etymology Dictionary.
- ↑ γλαυκός in Liddell and Scott.
- ↑ Leffler, CT; Schwartz, SG; Giliberti, FM; Young, MT; Bermudez, D (2015). "What was Glaucoma Called Before the 20th Century?". Ophthalmology and Eye Diseases. 7: 21–33. doi:10.4137/OED.S32004. ISSN 1179-1721. PMC 4601337. PMID 26483611. Archived from the original on 23 April 2016.
- ↑ Leffler CT, Schwartz SG, Hadi TM, Salman A, Vasuki V (2015). "The early history of glaucoma: the glaucous eye (800 BC to 1050 AD)". Clinical Ophthalmology. 9: 207–15. doi:10.2147/OPTH.S77471. PMC 4321651. PMID 25673972. Archived from the original on 26 February 2015.
- ↑ Sean K Wang, Robert T Chang (2014). "An emerging treatment option for glaucoma: Rho kinase inhibitors". Clin Ophthalmol. 8: 883–89. doi:10.2147/OPTH.S41000. PMC 4025933. PMID 24872673.
- ↑ 109.0 109.1 109.2 Sena DF, Lindsley K (2017). "Neuroprotection for treatment of glaucoma in adults". Cochrane Database Syst Rev. 1: CD006539. doi:10.1002/14651858.CD006539.pub4. PMC 5370094. PMID 28122126.
- ↑ Joy, J. E.; Watson Jr., S. J.; Benson Jr., J. A. (1999). Marijuana and Medicine: Assessing the Science Base. Nap.edu. doi:10.17226/6376. ISBN 978-0-309-07155-0. PMID 25101425. Retrieved 20 February 2014.
- ↑ Joy, J. E.; Watson Jr., S. J.; Benson Jr., J. A. (1999). Marijuana and Medicine: Assessing the Science Base (1999), Institute of Medicine, National Academies Press. Nap.edu. doi:10.17226/6376. ISBN 978-0-309-07155-0. PMID 25101425. Retrieved 22 June 2011.
- ↑ 112.0 112.1 "Complementary Therapy Assessment: Marijuana in the Treatment of Glaucoma". American Academy of Ophthalmology. Archived from the original on 24 July 2011. Retrieved 4 May 2011.
- ↑ "Complementary Therapy Assessments : American Academy of Ophthalmology". One.aao.org. Archived from the original on 24 July 2011. Retrieved 24 January 2011.
- ↑ Jampel H (February 2010). "American glaucoma society position statement: marijuana and the treatment of glaucoma". J. Glaucoma. 19 (2): 75–6. doi:10.1097/IJG.0b013e3181d12e39. PMID 20160576. S2CID 40362575.
External links
| Classification | |
|---|---|
| External resources |
- Glaucoma at Curlie
- GeneReview/NCBI/NIH/UW entry on Primary Congenital Glaucoma Archived 31 July 2020 at the Wayback Machine
- Pages with script errors
- CS1 maint: DOI inactive as of June 2020
- All pages needing factual verification
- Wikipedia articles needing factual verification from December 2013
- Articles with invalid date parameter in template
- Articles containing Ancient Greek (to 1453)-language text
- Use dmy dates from January 2018
- Use American English from December 2017
- All Wikipedia articles written in American English
- Wikipedia articles needing clarification from November 2018
- All articles with unsourced statements
- Articles with unsourced statements from May 2020
- Articles with hatnote templates targeting a nonexistent page
- Articles with unsourced statements from May 2009
- Articles with Curlie links
- Webarchive template wayback links
- Glaucoma
- Blindness
- Articles containing video clips
- RTT
- RTTNEURO

