Brimonidine
 | |
| Names | |
|---|---|
| Pronunciation | /brɪˈmoʊnɪdiːn/ bri-MOH-nid-een |
| Trade names | Alphagan, Mirvaso, Lumify, others |
| |
| Clinical data | |
| Main uses | Glaucoma, rosacea.[1][2] |
| Side effects | Itchiness, redness, dry mouth[1] |
| Pregnancy category |
|
| Routes of use | topical (eye drop, gel) |
| Defined daily dose | 0.1 to 0.2 ml (eye drops)[3] |
| External links | |
| AHFS/Drugs.com | Topical: Monograph Eye: Monograph |
| MedlinePlus | a601232 |
| Legal | |
| License data |
|
| Legal status |
|
| Pharmacokinetics | |
| Metabolism | Primarily liver |
| Elimination half-life | 3 hours (ocular), 12 hours (topical) |
| Chemical and physical data | |
| Formula | C11H10BrN5 |
| Molar mass | 292.135 g·mol−1 |
| 3D model (JSmol) | |
| Melting point | 252 °C (486 °F) |
| |
| |
Brimonidine is a medication used to treat open-angle glaucoma, ocular hypertension, and rosacea.[1][2] In rosacea it improves the redness.[2] It is used as eye drops or applied to the skin.[1][2]
Common side effects when used in the eyes include itchiness, redness, and a dry mouth.[1] Common side effects when used on the skin include redness, burning, and headaches.[2] More significant side effects may include allergic reactions and low blood pressure.[2][1] Use in pregnancy appears to be safe.[2][1] When applied to the eye it works by decreasing the amount of aqueous humor made while increasing the amount that drains from the eye.[1] When applied to the skin it works by causing blood vessels to contract.[2]
Brimonidine was patented in 1972 and came into medical use in 1996.[4] It is available as a generic medication.[5] One milliliter in the United Kingdom costs the NHS about 1.10 £ as of 2019.[5] In the United States the wholesale cost of this amount is about US$0.60.[6] In 2017, it was the 167th most commonly prescribed medication in the United States, with more than three million prescriptions.[7][8]
Medical uses
Eye pressure
Brimonidine is indicated for the lowering of intraocular pressure in people with open-angle glaucoma or ocular hypertension. It is one of the active ingredient of brimonidine/timolol.
A 2017 Cochrane review found insufficient evidence to determine if brimonidine slows optic nerve damage.[9]
Rosacea
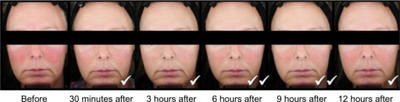
In 2013, the FDA approved topical application of brimonidine 0.33% gel for persistent facial redness of rosacea.
Dosage
The defined daily dose is 0.1 ml (once daily), or 0.2 ml (twice daily) as eye drops.[3]
Mechanism of action
Brimonidine is an α2 adrenergic agonist.[1]
α2 agonists, through the activation of a G protein-coupled receptor, inhibit the activity of adenylate cyclase. This reduces cAMP and hence aqueous humour production by the ciliary body.
Peripheral α2 agonist activity results in vasoconstriction of blood vessels (as opposed to central α2 agonist activity that decreases sympathetic tone, as can be seen by the medication clonidine). This vasoconstriction may explain the acute reduction in aqueous humor flow. The increased uveoscleral outflow from prolonged use may be explained by increased prostaglandin release due to α adrenergic stimulation. This may lead to relaxed ciliary muscle and increased uveoscleral outflow.[10]
Society and culture
Cost
One milliliter in the United Kingdom costs the NHS about 1.13 £ as of 2019.[5] In the United States the wholesale cost of this amount is about US$0.60.[6] In 2017, it was the 167th most commonly prescribed medication in the United States, with more than three million prescriptions.[7][8]
-
Costs (US)
-
Prescriptions (US)
A generic version of the skin gel was first approved in 2021 in the USA.[11]
Names
It is sold under the brand names Alphagan, Alphagan-P, Mirvaso Lumify, and others.
Over the counter
In July 2018, Bausch and Lomb began to market over the counter (OTC) eye drops, using brimonidine's tartrate formulation in a concentration of 0.025%, as an ophthalmic vasoconstrictor under the brand name Lumify. Intended to relieve redness in the sclerae of the eyes for periods of up to eight hours at a time through its vasoconstrictive effects, Lumify was marketed as an alternative to Visine, the brand of tetrahydrozoline hydrochloride solution most commonly used for that purpose.[citation needed]
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 1.6 1.7 1.8 "Brimonidine Tartrate eent Monograph for Professionals". Drugs.com. American Society of Health-System Pharmacists. Archived from the original on 14 May 2016. Retrieved 17 March 2019.
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 2.6 2.7 "Brimonidine Tartrate topical Monograph for Professionals". Drugs.com. American Society of Health-System Pharmacists. Archived from the original on 13 May 2021. Retrieved 17 March 2019.
- ↑ 3.0 3.1 "WikiProjectMed:Translation task force/RTT(Simplified)L - WikiProjectMed". mdwiki.org. Archived from the original on 30 May 2021. Retrieved 10 September 2020.
- ↑ Fischer, Jnos; Ganellin, C. Robin (2006). Analogue-based Drug Discovery. John Wiley & Sons. p. 550. ISBN 9783527607495. Archived from the original on 2019-03-06. Retrieved 2019-03-03.
- ↑ 5.0 5.1 5.2 British national formulary : BNF 76 (76 ed.). Pharmaceutical Press. 2018. p. 1153. ISBN 9780857113382.
- ↑ 6.0 6.1 "NADAC as of 2019-02-27". Centers for Medicare and Medicaid Services. Archived from the original on 2019-03-06. Retrieved 3 March 2019.
- ↑ 7.0 7.1 "The Top 300 of 2020". ClinCalc. Archived from the original on 12 February 2021. Retrieved 11 April 2020.
- ↑ 8.0 8.1 "Brimonidine Tartrate - Drug Usage Statistics". ClinCalc. Archived from the original on 26 February 2021. Retrieved 11 April 2020.
- ↑ Sena DF, Lindsley K (January 2017). "Neuroprotection for treatment of glaucoma in adults". The Cochrane Database of Systematic Reviews. 1: CD006539. doi:10.1002/14651858.CD006539.pub4. PMC 5370094. PMID 28122126.
- ↑ Toris CB, Camras CB, Yablonski ME (July 1999). "Acute versus chronic effects of brimonidine on aqueous humor dynamics in ocular hypertensive patients". American Journal of Ophthalmology. 128 (1): 8–14. doi:10.1016/s0002-9394(99)00076-8. PMID 10482088.
- ↑ Research, Center for Drug Evaluation and (10 February 2022). "2021 First Generic Drug Approvals". FDA. Archived from the original on 21 June 2022. Retrieved 22 October 2022.
External links
| Identifiers: |
|
|---|
- "Brimonidine". Drug Information Portal. U.S. National Library of Medicine. Archived from the original on 2021-05-16. Retrieved 2020-04-17.
- "Brimonidine tartrate". Drug Information Portal. U.S. National Library of Medicine. Archived from the original on 2020-06-11. Retrieved 2020-04-17.
- Oh DJ, Chen JL, Vajaranant TS, Dikopf MS (January 2019). "Brimonidine tartrate for the treatment of glaucoma". Expert Opin Pharmacother. 20 (1): 115–122. doi:10.1080/14656566.2018.1544241. PMID 30407890.
- Pages using duplicate arguments in template calls
- Chem-molar-mass both hardcoded and calculated
- Chemical articles with unknown parameter in Infobox drug
- Chemical articles without CAS registry number
- Articles without EBI source
- Chemical pages without ChemSpiderID
- Chemical pages without DrugBank identifier
- Articles without KEGG source
- Articles without UNII source
- Drugs missing an ATC code
- Drug has EMA link
- Drugboxes which contain changes to watched fields
- All articles with unsourced statements
- Articles with unsourced statements from April 2020
- Articles with invalid date parameter in template
- AbbVie brands
- Alpha-adrenergic agonists
- Imidazolines
- Organobromides
- Ophthalmology drugs
- Quinoxalines
- RTT

