Oxyfedrine
Jump to navigation
Jump to search
 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | International Drug Names |
| ATC code | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
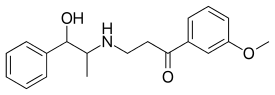
| Formula | C19H23NO3 |
| Molar mass | 313.397 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Oxyfedrine is a vasodilator and a β adrenoreceptor agonist. It was found to depress the tonicity of coronary vessels, improve myocardial metabolism (so that heart can sustain hypoxia better) and also exert a positive chronotropic and inotropic effects, thereby not precipitating angina pectoris. The latter property (positive chronotropic and inotropic effects) is particularly important, because other vasodilators used in angina may be counter productive causing coronary steal phenomenon.
Synergistic effects with antibiotics have been suggested.[1]
Synthesis

Mannich condensation of PPA [14838-15-4] (1) with formaldehyde and m-acetanisole (3-Acetylanisole) [586-37-8] (2) yields oxyfedrine (3).
References
- ^ Mazumdar K, Dutta NK, Kumar KA, Dastidar SG (April 2005). "In vitro and in vivo synergism between tetracycline and the cardiovascular agent oxyfedrine HCl against common bacterial strains". Biological & Pharmaceutical Bulletin. 28 (4): 713–7. doi:10.1248/bpb.28.713. PMID 15802815.
- ^ Thiele Kurt, U.S. patent 3,225,095 (1965 to Degussa).
Categories:
- Articles with short description
- Short description matches Wikidata
- Articles without EBI source
- Chemical pages without DrugBank identifier
- Multiple chemicals in Infobox drug
- Multiple chemicals in an infobox that need indexing
- Chemical articles with multiple PubChem CIDs
- Drugs with no legal status
- Drugboxes which contain changes to watched fields
- Vasodilators
- Phenol ethers
- Phenylethanolamines
- Substituted amphetamines
- Aromatic ketones
- All stub articles
- Cardiovascular system drug stubs