Tolterodine
 | |
| Names | |
|---|---|
| Trade names | Detrol, Detrusitol, others |
| |
| Clinical data | |
| Pregnancy category |
|
| Routes of use | By mouth |
| Defined daily dose | 4 mg[1] |
| External links | |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a699026 |
| Legal | |
| License data |
|
| Legal status | |
| Pharmacokinetics | |
| Bioavailability | 77% |
| Protein binding | Approximately 96.3% |
| Elimination half-life | 1.9–3.7 hours |
| Chemical and physical data | |
| Formula | C22H31NO |
| Molar mass | 325.496 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Tolterodine, sold under the brand names Detrol among others, is medication used to treat frequent urination, urinary incontinence, or urinary urgency.[2] Effects are seen within an hour.[3] It is taken by mouth.[3]
Common side effects include headache, dry mouth, constipation, and dizziness.[3] Serious side effects may include angioedema, urinary retention, and QT prolongation.[3] Use in pregnancy and breastfeeding are of unclear safety.[2][4] It works by blocking muscarinic receptors in the bladder thus decreasing bladder contractions.[3]
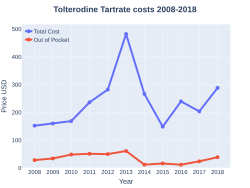
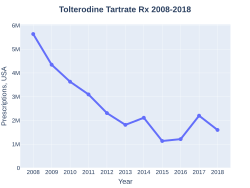
Tolterodine was approved for medical use in 1998.[3] It is available as a generic medication.[2] A month supply costs the NHS about GB£2.09 per month as of 2019.[2] In the United States the wholesale cost of this amount is about US$37.16.[5] In 2017, it was the 229th most commonly prescribed medication in the United States, with more than two million prescriptions.[6][7]
Medical uses
Detrusor overactivity (DO, contraction of the muscular bladder wall) is the most common form of urinary incontinence (UI) in older adults. It is characterized by uninhibited bladder contractions causing an uncontrollable urge to void. Urinary frequency, urge incontinence and nocturnal incontinence occur. Abnormal bladder contractions that coincide with the urge to void can be measured by urodynamic studies. Treatment is bladder retraining,[8] pelvic floor therapy or with drugs that inhibit bladder contractions such as oxybutynin and tolterodine.
Dosage
The defined daily dose is 4 mg by mouth.[1]
Side effects
Known side effects:
- Dry mouth
- Decreased gastric motility (upset stomach)
- Headache
- Constipation
- Dry eyes
- Sleepiness
- Urinary retention
The following reactions have been reported in patients who have taken tolterodine since it has become available:
- Allergic reactions including swelling
- Rapid heartbeat or abnormal heartbeat
- Accumulation of fluid in the arms and legs
- Hallucinations
Tolterodine is not recommended for use in people with myasthenia gravis and angle closure glaucoma.
Pharmacology
Tolterodine acts on M2 and M3[9] subtypes of muscarinic receptors whereas older antimuscarinic treatments for overactive bladder act more specifically on M3 receptors.
Tolterodine, although it acts on all types of receptors, has fewer side effects than oxybutynin (M3 and M1 selective, but more so in the parotid than in the bladder) as tolterodine targets the bladder more than other areas of the body. This means that less drug needs to be given daily (due to efficient targeting of the bladder) and so there are fewer side effects.[citation needed]
Brand names
It is marketed by Pfizer in Canada and the United States by its brand name Detrol. In Egypt it is also found under the trade names Tolterodine by Sabaa and Incont L.A. by Adwia.
Society and culture
Cost
A month supply costs the NHS about GB£2.09 per month as of 2019.[2] In the United States the wholesale cost of this amount is about US$37.16.[5] In 2017, it was the 229th most commonly prescribed medication in the United States, with more than two million prescriptions.[6][7]
-
Tolterodine costs (US)
-
Tolterodine prescriptions (US)
References
- ↑ 1.0 1.1 "WHOCC - ATC/DDD Index". www.whocc.no. Archived from the original on 12 December 2020. Retrieved 9 September 2020.
- ↑ 2.0 2.1 2.2 2.3 2.4 British national formulary : BNF 76 (76 ed.). Pharmaceutical Press. 2018. p. 762. ISBN 9780857113382.
- ↑ 3.0 3.1 3.2 3.3 3.4 3.5 "Tolterodine Tartrate Monograph for Professionals". Drugs.com. American Society of Health-System Pharmacists. Archived from the original on 27 March 2019. Retrieved 3 March 2019.
- ↑ "tolterodine Use During Pregnancy". Drugs.com. Archived from the original on 6 March 2019. Retrieved 3 March 2019.
- ↑ 5.0 5.1 "NADAC as of 2019-02-27". Centers for Medicare and Medicaid Services. Archived from the original on 2019-03-06. Retrieved 3 March 2019.
- ↑ 6.0 6.1 "The Top 300 of 2020". ClinCalc. Archived from the original on 18 March 2020. Retrieved 11 April 2020.
- ↑ 7.0 7.1 "Tolterodine Tartrate - Drug Usage Statistics". ClinCalc. Archived from the original on 4 July 2020. Retrieved 11 April 2020.
- ↑ "Bladder retraining". Interstitial Cystitis Association. Archived from the original on 28 July 2018. Retrieved 6 June 2018.
- ↑ [1] Archived November 26, 2012, at the Wayback Machine
External links
| External sites: | |
|---|---|
| Identifiers: |
|
- Pages using duplicate arguments in template calls
- Webarchive template wayback links
- Chemical articles with unknown parameter in Infobox drug
- Chemical articles without CAS registry number
- Articles without EBI source
- Chemical pages without ChemSpiderID
- Chemical pages without DrugBank identifier
- Articles without KEGG source
- Articles without UNII source
- Drugs missing an ATC code
- Drugboxes which contain changes to watched fields
- All articles with unsourced statements
- Articles with unsourced statements from July 2012
- Articles with invalid date parameter in template
- Muscarinic antagonists
- Pfizer brands
- Phenols
- RTT
- Diisopropylamino compounds
- Benzhydryl compounds