Reproterol
Jump to navigation
Jump to search
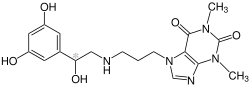 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | International Drug Names |
| Routes of administration | Inhalation (MDI), IV |
| ATC code | |
| Legal status | |
| Legal status | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.053.579 |
| Chemical and physical data | |
| Formula | C18H23N5O5 |
| Molar mass | 389.412 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Reproterol is a short-acting[1] β2 adrenoreceptor agonist used in the treatment of asthma.[2]
It was patented in 1965 and came into medical use in 1977.[3]
Stereochemistry
Reproterol contains a stereocenter and is chiral. There are thus two enantiomers, the (R)-form and the (S)-form. The commercial preparations contain the drug as a racemate, an equal mixture of the two enantiomers.[4]
| Enantiomers of reproterol | |
|---|---|
 (R)-Reproterol CAS number: 210710-33-1 |
 (S)-Reproterol CAS number: 210710-34-2 |
References
- ^ Küpper T, Goebbels K, Kennes LN, Netzer NC (December 2012). "Cromoglycate, reproterol, or both--what's best for exercise-induced asthma?". Sleep & Breathing = Schlaf & Atmung. 16 (4): 1229–35. doi:10.1007/s11325-011-0638-2. PMID 22198635. S2CID 10032756.
- ^ Virchow JC (1999). "Reproterol: beta-2-agonist, theophylline, or both?". Respiration; International Review of Thoracic Diseases. 66 (3): 210–1. doi:10.1159/000029379. PMID 10364735. S2CID 203732706.
- ^ Fischer J, Ganellin CR (2006). Analogue-based Drug Discovery. John Wiley & Sons. p. 54X. ISBN 9783527607495.
- ^ Rote Liste 2017 – Arzneimittelverzeichnis für Deutschland (einschließlich EU-Zulassungen und bestimmter Medizinprodukte). Vol. 57. Frankfurt/Main: Rote Liste Service GmbH. 2017. p. 196. ISBN 978-3-946057-10-9.
Categories:
- Articles with short description
- Short description matches Wikidata
- Articles with changed ChemSpider identifier
- Articles with changed EBI identifier
- ECHA InfoCard ID from Wikidata
- Articles with changed InChI identifier
- Chemical pages without DrugBank identifier
- Drugs with no legal status
- Drugboxes which contain changes to verified fields
- Antiasthmatic drugs
- Beta-adrenergic agonists
- Xanthines
- Phenylethanolamines
- Resorcinols
- All stub articles
- Respiratory system drug stubs