Pethidine
 | |
 | |
| Names | |
|---|---|
| Trade names | Demerol, others |
| Other names | Meperidine (USAN US) |
| |
| Clinical data | |
| Drug class | Opioid[1] |
| Main uses | Moderate to severe pain[1] |
| Side effects | Sleepiness, seizures, agitation, vomiting, constipation, decreased effort to breath[1] |
| Dependence risk | High |
| Pregnancy category |
|
| Routes of use | By mouth, IV, IM, IT,[2] SC, epidural[3] |
| Onset of action | < 1 hr[1] |
| Duration of action | 2 to 4 hrs[1] |
| External links | |
| AHFS/Drugs.com | Monograph |
| Legal | |
| Legal status |
|
| Pharmacokinetics | |
| Bioavailability | 50–60% (Oral), 80–90% (Oral, in cases of hepatic impairment) |
| Protein binding | 65–75% |
| Metabolism | Liver |
| Elimination half-life | 2.5–4 hours, 7–11 hours (liver disease) |
| Excretion | Kidney |
| Chemical and physical data | |
| Formula | C15H21NO2 |
| Molar mass | 247.338 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Pethidine, also known as meperidine and sold under the brand name Demerol among others, is a pain medication of the opioid type.[1] Specifically it may be used for moderate to severe pain for a few days.[1] It is not a first line opioid and long term use is not recommended.[1] It can be used mouth, or by injection into a muscle, or vein.[1] It; however, is not very effective by mouth and such use is associated with greater toxicity.[1] Onset of effects is within an hour and last for up to 4 hours.[1]
Common side effects include sleepiness, seizures, agitation, vomiting, constipation, and decreased effort to breath.[1] Other serious complications include heart arrhythmias, psychosis, addiction, low blood pressure, and serotonin syndrome.[1] Prolonged use during pregnancy may result in neonatal abstinence syndrome in the baby.[4] Pethidine works by binding and activating the opiate receptor.[1]
Pethidine was patented in 1937 and approved for medical use in 1943.[5] In the 20th century it was a commonly used opioid; in 1975, 60% of doctors prescribed it for acute pain and 22% for chronic severe pain.[6] In the 2000s its use has become less common.[7] It is avaliable as a generic medication.[8] In the United Kingdom the injectable formulation costs the NHS about 0.5 pounds per 50 mg in 2020.[8]
Medical uses
Pethidine was the most widely used opioid in labour and delivery[9] but has fallen out of favour in some countries such as the United States, due to drug interactions (especially with serotonergics) and its neurotoxic metabolite, norpethidine.[10] It was a commonly used in the United Kingdom during labour, but has been superseded somewhat by diamorphine (heroin) and other opioids (e.g. hydromorphone) to avoid serotonin interactions since the mid-2000s.[11]
While less commonly used for pain it may still be used occasionally for shivering that occurs after surgery.[7]
Before 2003 it was on the World Health Organization's List of Essential Medicines.[12][13]
Side effects
The side effects of pethidine administration are primarily those of the opioids as a class: nausea, vomiting, dizziness, diaphoresis, urinary retention, and constipation. Due to moderate stimulant effects mediated by dopamine and norepinephrine, sedation is less likely compared to other opioids. Unlike other opioids, it does not cause miosis because of its anticholinergic properties. Overdose can cause muscle flaccidity, respiratory depression, obtundation, cold and clammy skin, hypotension, and coma. A narcotic antagonist such as naloxone is indicated to reverse respiratory depression and other effects of pethidine. Serotonin syndrome has occurred in patients receiving concurrent antidepressant therapy with selective serotonin reuptake inhibitors (SSRIs) or monoamine oxidase inhibitors, or other medication types (see Interactions below). Convulsive seizures sometimes observed in patients receiving parenteral pethidine on a chronic basis have been attributed to accumulation in plasma of the metabolite norpethidine (normeperidine). Fatalities have occurred following either oral or intravenous pethidine overdose.[14][15]
Interactions
Pethidine has serious interactions that can be dangerous with monoamine oxidase inhibitors (e.g., furazolidone, isocarboxazid, moclobemide, phenelzine, procarbazine, selegiline, tranylcypromine). Such patients may suffer agitation, delirium, headache, convulsions, and/or hyperthermia. Fatal interactions have been reported including the death of Libby Zion.[16] Seizures may develop when tramadol is given intravenously following, or with, pethidine.[17] It can interact as well with SSRIs and other antidepressants, antiparkinson agents, migraine therapy, stimulants and other agents causing serotonin syndrome. It is thought to be caused by an increase in cerebral serotonin concentrations. It is probable that pethidine can also interact with a number of other medications, including muscle relaxants, benzodiazepines, and ethanol.
Mechanism of action
Like morphine, pethidine exerts its analgesic effects by acting as an agonist at the μ-opioid receptor.[18]
Pethidine is often employed in the treatment of postanesthetic shivering. The pharmacologic mechanism of this antishivering effect is not fully understood,[19] but it may involve the stimulation of κ-opioid receptors.[20]
Pethidine has structural similarities to atropine and other tropane alkaloids and may have some of their effects and side effects.[21] In addition to these opioidergic and anticholinergic effects, it has local anesthetic activity related to its interactions with sodium ion channels.
Pethidine's apparent in vitro efficacy as an antispasmodic agent is due to its local anesthetic effects. It does not have antispasmodic effects in vivo.[22] Pethidine also has stimulant effects mediated by its inhibition of the dopamine transporter (DAT) and norepinephrine transporter (NET). Because of its DAT inhibitory action, pethidine will substitute for cocaine in animals trained to discriminate cocaine from saline.[23]
Several analogs of pethidine such as 4-fluoropethidine have been synthesized that are potent inhibitors of the reuptake of the monoamine neurotransmitters dopamine and norepinephrine via DAT and NET.[24][25] It has also been associated with cases of serotonin syndrome, suggesting some interaction with serotonergic neurons, but the relationship has not been definitively demonstrated.[23][25][26][27]
It is more lipid-soluble than morphine, resulting in a faster onset of action. Its duration of clinical effect is 120–150 minutes, although it is typically administered at 4– to 6-hour intervals. Pethidine has been shown to be less effective than morphine, diamorphine, or hydromorphone at easing severe pain, or pain associated with movement or coughing.[23][25][27]
Like other opioid drugs, pethidine has the potential to cause physical dependence or addiction. It may be more likely to be abused than other prescription opioids, perhaps because of its rapid onset of action.[28] When compared with oxycodone, hydromorphone, and placebo, pethidine was consistently associated with more euphoria, difficulty concentrating, confusion, and impaired psychomotor and cognitive performance when administered to healthy volunteers.[29] The especially severe side effects unique to pethidine among opioids—serotonin syndrome, seizures, delirium, dysphoria, tremor—are primarily or entirely due to the action of its metabolite, norpethidine.[25][27]
Pharmacokinetics
Pethidine is quickly hydrolysed in the liver to pethidinic acid and is also demethylated to norpethidine, which has half the analgesic activity of pethidine but a longer elimination half-life (8–12 hours);[30] accumulating with regular administration, or in kidney failure. Norpethidine is toxic and has convulsant and hallucinogenic effects. The toxic effects mediated by the metabolites cannot be countered with opioid receptor antagonists such as naloxone or naltrexone, and are probably primarily due to norpethidine's anticholinergic activity probably due to its structural similarity to atropine, though its pharmacology has not been thoroughly explored. The neurotoxicity of pethidine's metabolites is a unique feature of pethidine compared to other opioids. Pethidine's metabolites are further conjugated with glucuronic acid and excreted into the urine.
Pethidine is the prototype of a large family of analgesics including the pethidine 4-phenylpiperidines (piminodine, anileridine and others), the prodines (alphaprodine, MPPP, etc.), bemidones (ketobemidone, etc.) and others more distant, including diphenoxylate and analogues.[31]
History
It was developed as a potential anticholinergic agent by the German chemist Otto Eisleb, its analgesic properties were first recognized by Otto Schaumann while working for IG Farben, Germany.[32]
Compared with morphine, pethidine was initially thought to be safer, carry a lower risk of addiction, and to be superior in treating the pain associated with biliary spasm or renal colic due to its putative anticholinergic effects.[6] These were later discovered to be incorrect, as it carries an least equal risk of addiction, possesses no advantageous effects on biliary spasm or renal colic compared to other opioids, and due to its toxic metabolite, norpethidine, it is more toxic than other opioids—especially during long-term use.[6] The norpethidine metabolite was found to have serotonergic effects, so pethidine could, unlike most opioids, contribute to serotonin syndrome.[6][33]
Society and culture
Recreational use
In data from the U.S. Drug Abuse Warning Network, mentions of hazardous or harmful use of pethidine declined between 1997 and 2002, in contrast to increases for fentanyl, hydromorphone, morphine, and oxycodone.[34] The number of dosage units of pethidine reported lost or stolen in the U.S. increased 16.2% between 2000 and 2003, from 32,447 to 37,687.[35]
This article uses the terms "hazardous use", "harmful use", and "dependence" in accordance with Lexicon of alcohol and drug terms Archived 2004-07-04 at the Wayback Machine published by the World Health Organization (WHO) in 1994.[36] In WHO usage, the first two terms replace the term "abuse" and the third term replaces the term "addiction".[36][37]
Synthesis
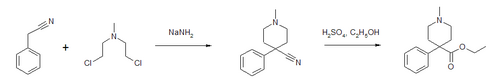
Pethidine can be produced in a two-step synthesis. The first step is reaction of benzyl cyanide and chlormethine in the presence of sodium amide to form a piperidine ring. The nitrile is then converted to an ester.[38]
Control
Pethidine is in Schedule II of the Controlled Substances Act 1970 of the United States as a Narcotic with ACSCN 9230 with a 6250 kilo aggregate manufacturing quota as of 2014. The free base conversion ratio for salts includes 0.87 for the hydrochloride and 0.84 for the hydrobromide. The A, B, and C intermediates in production of pethidine are also controlled, with ACSCN being 9232 for A (with a 6 gram quota) and 9233 being B (quota of 11 grams) and 9234 being C (6 gram quota).[39] It is listed under the Single Convention for the Control of Narcotic Substances 1961 and is controlled in most countries in the same fashion as is morphine.
See also
- Libby Zion Law (a case involving phenelzine and pethidine)
References
- ↑ 1.00 1.01 1.02 1.03 1.04 1.05 1.06 1.07 1.08 1.09 1.10 1.11 1.12 1.13 "Meperidine Hydrochloride Monograph for Professionals". Drugs.com. Archived from the original on 28 August 2021. Retrieved 10 November 2020.
- ↑ Ngan Kee, WD (April 1998). "Intrathecal pethidine: pharmacology and clinical applications". Anaesthesia and Intensive Care. 26 (2): 137–46. doi:10.1177/0310057X9802600202. PMID 9564390.
- ↑ Ngan Kee, WD (June 1998). "Epidural pethidine: pharmacology and clinical experience". Anaesthesia and Intensive Care. 26 (3): 247–55. doi:10.1177/0310057X9802600303. PMID 9619217.
- ↑ "Meperidine Use During Pregnancy". Drugs.com. Archived from the original on 28 October 2020. Retrieved 10 November 2020.
- ↑ Fischer, Jnos; Ganellin, C. Robin (2006). Analogue-based Drug Discovery. John Wiley & Sons. p. 526. ISBN 9783527607495. Archived from the original on 2021-04-15. Retrieved 2020-11-10.
- ↑ 6.0 6.1 6.2 6.3 Latta, KS; Ginsberg, B; Barkin, RL (January–February 2002). "Meperidine: a critical review". American Journal of Therapeutics. 9 (1): 53–68. doi:10.1097/00045391-200201000-00010. PMID 11782820. S2CID 23410891.
- ↑ 7.0 7.1 Murray, Michael J.; Rose, Steven H.; Wedel, Denise J.; Wass, C. Thomas; Harrison, Barry A.; Mueller, Jeff T.; Trentman, Terence L. (2014). Faust's Anesthesiology Review E-Book. Elsevier Health Sciences. p. 169. ISBN 978-1-4377-0367-2. Archived from the original on 2021-08-28. Retrieved 2020-11-10.
- ↑ 8.0 8.1 BNF 79 : March 2020. London: Royal Pharmaceutical Society. 2020. p. 481. ISBN 9780857113658.
- ↑ "Parenteral opioids for labor pain relief: A systematic review - American Journal of Obstetrics & Gynecology". www.ajog.org. Archived from the original on 2018-09-16. Retrieved 2015-07-11.
- ↑ Rossi, S, ed. (2013). Australian Medicines Handbook (2013 ed.). Adelaide: The Australian Medicines Handbook Unit Trust. ISBN 978-0-9805790-9-3.
- ↑ "Pain relief in labour - Pregnancy and baby guide - NHS Choices". www.nhs.uk. Archived from the original on 2015-06-12. Retrieved 2015-06-20.
- ↑ "Essential Medicines WHO Model List (revised April 2002)" (PDF). apps.who.int (12th ed.). Geneva, Switzerland: World Health Organization. April 2002. Archived (PDF) from the original on 8 December 2017. Retrieved 6 September 2017.
- ↑ "Essential Medicines WHO Model List (revised April 2003)" (PDF). apps.who.int (13th ed.). Geneva, Switzerland: World Health Organization. April 2003. Archived (PDF) from the original on 27 June 2016. Retrieved 6 September 2017.
- ↑ Baselt, R. (2008). Disposition of Toxic Drugs and Chemicals in Man (8 ed.). Foster City, CA: Biomedical Publications. pp. 911-914.
- ↑ Package insert for meperidine hydrochloride, Boehringer Ingelheim, Ridgefield, CT, 2005.
- ↑ Brody, Jane (February 27, 2007). "A Mix of Medicines That Can Be Lethal". New York Times. Archived from the original on November 13, 2013. Retrieved 2009-02-13.
The death of Libby Zion, an 18-year-old college student, in a New York hospital on March 5, 1984, led to a highly publicized court battle and created a cause célèbre over the lack of supervision of inexperienced and overworked young doctors. But only much later did experts zero in on the preventable disorder that apparently led to Ms. Zion’s death: a form of drug poisoning called serotonin syndrome.
- ↑ "Serious Reactions with Tramadol: Seizures and Serotonin Syndrome". medsafe.govt.nz (Prescriber Update 28(1) ed.). New Zealand Pharmacovigilance Centre, Dunedin: New Zealand Medicines and Medical Devices Safety Authority. October 2007. Archived from the original on 7 November 2019. Retrieved 7 November 2019.
- ↑ Bronwen Bryant & Kathleen Knights (2010). Pharmacology for Health Professionals, 3rd Edition. Chatswood: Mosby Australia. ISBN 978-0-7295-3929-6.
- ↑ Koczmara, C; Perri, D; Hyland, S; Rousseaux, L (2005). "Meperidine (Demerol®) safety issues" (PDF). Official Journal of the Canadian Association of Critical Care Nurses. 16 (1): 8–12. ISSN 1201-2580. Archived (PDF) from the original on 2014-01-12. Retrieved 2014-01-11.
- ↑ Laurence, Brunton (2010). Goodman & Gilman's pharmacological basis of therapeutics (12th ed.). McGraw-Hill. p. 549. ISBN 978-0071624428.
- ↑ "Archive copy". Archived from the original on 2007-09-30. Retrieved 2007-09-01.
{{cite web}}: CS1 maint: archived copy as title (link) - ↑ Wagner, Larry E., II; Michael Eaton; Salas S. Sabnis; Kevin J. Gingrich (November 1999). "Meperidine and Lidocaine Block of Recombinant Voltage-Dependent Na+ Channels: Evidence that Meperidine is a Local Anesthetic". Anesthesiology. 91 (5): 1481–1490. doi:10.1097/00000542-199911000-00042. PMID 10551601. S2CID 34806974. Archived from the original on 2021-08-28. Retrieved 2019-11-24.
- ↑ 23.0 23.1 23.2 Izenwasser, Sari; Amy Hauck Newman; Brian M. Cox; Jonathan L. Katz (January–February 1996). "The cocaine-like behavioral effects of meperidine are mediated by activity at the dopamine transporter". European Journal of Pharmacology. 297 (1–2): 9–17. doi:10.1016/0014-2999(95)00696-6. PMID 8851160.
- ↑ Lomenzo, Stacey A.; Jill B. Rhoden; Sari Izzenwasser; Dean Wade; Theresa Kopajtic; Jonathan L. Katz; Mark L. Trudell (2005-03-05). "Synthesis and Biological Evaluation of Meperdine Analogs at Monoamine Transporters". Journal of Medicinal Chemistry. 48 (5): 1336–1343. doi:10.1021/jm0401614. PMID 15743177. Archived from the original on 2020-08-03. Retrieved 2019-11-24.
- ↑ 25.0 25.1 25.2 25.3 "Demerol: Is It the Best Analgesic?" (PDF), Pennsylvania Patient Safety Reporting Service Patient Safety Advisory, 3 (2), June 2006, archived from the original (PDF) on 2013-06-20, retrieved 2013-04-15
- ↑ Davis, Sharon (August 2004). "Use of pethidine for pain management in the emergency department: a position statement of the NSW Therapeutic Advisory Group" (PDF). New South Wales Therapeutic Advisory Group. Archived from the original (PDF) on 2006-10-09. Retrieved 2007-01-17.
- ↑ 27.0 27.1 27.2 Latta, Kenneth S.; Brian Ginsberg; Robert L. Barkin (January–February 2002). "Meperidine: A Critical Review". American Journal of Therapeutics. 9 (1): 53–68. doi:10.1097/00045391-200201000-00010. PMID 11782820. S2CID 23410891.
- ↑ "In Brief" (PDF). NPS Radar. National Prescribing Service. December 2005. Archived from the original (PDF) on 2009-10-28. Retrieved 2009-12-22.
- ↑ Walker, Diana J.; James P. Zacny (June 1999). "Subjective, Psychomotor, and Physiological Effects of Cumulative Doses of Opioid µ Agonists in Healthy Volunteers". The Journal of Pharmacology and Experimental Therapeutics. 289 (3): 1454–1464. PMID 10336539.
- ↑ Molloy, A (2002). "Does pethidine still have a place in therapy?" (PDF). Australian Prescriber. 25 (1): 12–13. doi:10.18773/austprescr.2002.008. Archived from the original (PDF) on 2013-08-20.
- ↑ Morphine and Allied Drugs, AK Reynolds & LO Randall, U of Toronto Press, Toronto 1957, and Oxford University Press (London) No ISBN given in book; pp. 273–319
- ↑ Michaelis, Martin; Schölkens, Bernward; Rudolphi, Karl (April 2007). "An anthology from Naunyn-Schmiedeberg's Archives of Pharmacology". Naunyn-Schmiedeberg's Archives of Pharmacology. 375 (2): 81–84. doi:10.1007/s00210-007-0136-z. PMID 17310263. S2CID 27774155.
- ↑ MacPherson RD, Duguid MD (2008). "Strategy to Eliminate Pethidine Use in Hospitals" (PDF). Journal of Pharmacy Practice and Research. 38 (2): 88–89. doi:10.1002/j.2055-2335.2008.tb00807.x. S2CID 71812645. Archived from the original (PDF) on 2014-02-15.
- ↑ Gilson AM, Ryan KM, Joranson DE, Dahl JL (2004). "A reassessment of trends in the medical use and abuse of opioid analgesics and implications for diversion control: 1997-2002". J Pain Symptom Manage. 28 (2): 176–188. CiteSeerX 10.1.1.387.1900. doi:10.1016/j.jpainsymman.2004.01.003. PMID 15276196.
- ↑ Joranson DE, Gilson AM (2005). "Drug crime is a source of abused pain medications in the United States". J Pain Symptom Manage. 30 (4): 299–301. doi:10.1016/j.jpainsymman.2005.09.001. PMID 16256890.
- ↑ 36.0 36.1 Babor T, Campbell R, Room R, Saunders J, eds. (1994). Lexicon of alcohol and drug terms. Geneva: World Health Organization. ISBN 978-92-4-154468-9.
- ↑ Lomenzo, SA; Rhoden, JB; Izenwasser, S; et al. (March 2005). [10.1021/jm0401614/suppl_file/jm0401614_s.pdf "Synthesis and biological evaluation of meperidine analogues at monoamine transporters"] (PDF). J. Med. Chem. 48 (5): 1336–43. doi:10.1021/jm0401614. PMID 15743177.
{{cite journal}}: Check|url=value (help) - ↑ Patent Appl. DE 679 281 IG Farben 1937.
- ↑ "Archive copy". Archived from the original on 2016-03-04. Retrieved 2016-02-26.
{{cite web}}: CS1 maint: archived copy as title (link)
External links
| Identifiers: |
|
|---|
- Pages using duplicate arguments in template calls
- CS1 maint: archived copy as title
- CS1 errors: URL
- Infobox drug with local INN variant
- Chemical articles with unknown parameter in Infobox drug
- Chemical articles without CAS registry number
- Articles without EBI source
- Chemical pages without ChemSpiderID
- Chemical pages without DrugBank identifier
- Articles without KEGG source
- Articles without UNII source
- Drugs missing an ATC code
- Drugboxes which contain changes to watched fields
- Articles with hatnote templates targeting a nonexistent page
- Webarchive template wayback links
- Analgesics
- Convulsants
- Ethyl esters
- Euphoriants
- Glycine receptor antagonists
- Kappa agonists
- Local anesthetics
- Mu-opioid agonists
- Muscarinic antagonists
- NMDA receptor antagonists
- Piperidines
- Serotonin-norepinephrine-dopamine reuptake inhibitors
- Sodium channel blockers
- Synthetic opioids
- RTT