Brompheniramine
 | |
| Names | |
|---|---|
| Trade names | Bromfed, Dimetapp, Bromfenex, and others |
| |
| Clinical data | |
| Drug class | Antihistamine[1] |
| Main uses | Common cold and allergic rhinitis[1] |
| Side effects | Sleepiness, restlessness, confusion, dry mouth, blurred vision[1] |
| Pregnancy category |
|
| Routes of use | By mouth |
| Duration of action | 48 hrs[1] |
| External links | |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a682545 |
| Legal | |
| Legal status | |
| Pharmacokinetics | |
| Metabolism | Liver |
| Elimination half-life | 24.9 ± 9.3 hours[3] |
| Excretion | Urine |
| Chemical and physical data | |
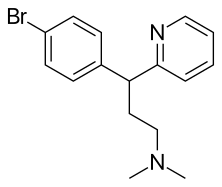
| Formula | C16H19BrN2 |
| Molar mass | 319.246 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Brompheniramine, sold under the brand name Dimetapp among others,[2] is an antihistamine used for symptoms of the common cold and allergic rhinitis, such as runny nose, itchy eyes, watery eyes, and sneezing.[1] Its use in children under 6 is not recommended.[4] It is taken by mouth.[1] Maximal effects occur around 6 hours and may may last for up to two days.[1]
Common side effects include sleepiness, restlessness, confusion, dry mouth, and blurred vision.[1] Other side effects may include urinary retention and seizures.[1] It is a first-generation antihistamine.[1]
Brompheniramine was patented in 1948 and came into medical use in 1955.[5] It is frequently sold in combination with other medications such as dextromethorphan, guaifenesin, or pseudoephedrine.[1] These combinations are available as generic medication and are relatively inexpensive.[6][7]
Side effects
Brompheniramine's effects on the cholinergic system may include side-effects such as drowsiness, sedation, dry mouth, dry throat, blurred vision, and increased heart rate. It is listed as one of the drugs of highest anticholinergic activity in a study of anticholinergenic burden, including long-term cognitive impairment.[8]
Pharmacology
Brompheniramine works by acting as an antagonist of histamine H1 receptors. It also functions as a moderately effective anticholinergic agent, and is likely an antimuscarinic agent similar to other common antihistamines such as diphenhydramine.
Brompheniramine is metabolised by cytochrome P450s.
The halogenated alkylamine antihistamines all exhibit optic isomerism and brompheniramine products contain racaemic brompheniramine maleate whereas dexbrompheniramine (Drixoral) is the dextrorotary (right-handed) stereoisomer.[citation needed]
Chemistry
Brompheniramine is part of a series of antihistamines including pheniramine (Naphcon) and its halogenated derivatives and others including fluorpheniramine, chlorpheniramine, dexchlorpheniramine (Polaramine), triprolidine (Actifed), and iodopheniramine. The halogenated alkylamine antihistamines all exhibit optical isomerism and brompheniramine products contain racemic brompheniramine maleate whereas dexbrompheniramine (Drixoral) is the dextrorotary (right-handed) stereoisomer.
Brompheniramine is an analog of chlorpheniramine. The only difference is that the chlorine atom in the benzene ring is replaced with a bromine atom. It is also synthesized in an analogous manner.[9][10]
History
Based on this[which?] knowledge, Arvid Carlsson and his colleagues, working at the Swedish company Astra AB, were able to derive the first marketed selective serotonin reuptake inhibitor, zimelidine, from brompheniramine.[11]
Society and culture
Cost
Brompheniramine has a U.S. cost as Dimetapp of $9.31 bottle.[7]
Names
Brand names include Bromfed, Dimetapp, Bromfenex, Dimetane, BPN, Lodrane. It is commonly marketed as its salt brompheniramine maleate.[citation needed]
See also
References
- ↑ 1.00 1.01 1.02 1.03 1.04 1.05 1.06 1.07 1.08 1.09 1.10 "Brompheniramine Monograph for Professionals". Drugs.com. Archived from the original on 21 January 2021. Retrieved 17 July 2021.
- ↑ 2.0 2.1 "Brompheniramine Uses, Side Effects & Warnings". Drugs.com. Archived from the original on 28 January 2021. Retrieved 25 March 2021.
- ↑ Simons FE, Frith EM, Simons KJ (December 1982). "The pharmacokinetics and antihistaminic effects of brompheniramine". The Journal of Allergy and Clinical Immunology. 70 (6): 458–64. doi:10.1016/0091-6749(82)90009-4. PMID 6128358.
- ↑ BNF (80 ed.). BMJ Group and the Pharmaceutical Press. September 2020 – March 2021. p. 312. ISBN 978-0-85711-369-6.
{{cite book}}: CS1 maint: date format (link) - ↑ Fischer, Jnos; Ganellin, C. Robin (2006). Analogue-based Drug Discovery. John Wiley & Sons. p. 546. ISBN 9783527607495. Archived from the original on 2021-05-12. Retrieved 2020-12-15.
- ↑ "Brompheniramine / Dextromethorphan / Pseudoephedrine Prices, Coupons & Savings Tips - GoodRx". GoodRx. Archived from the original on 17 May 2020. Retrieved 17 July 2021.
- ↑ 7.0 7.1 "Compare Dimetapp Prices". GoodRx. Archived from the original on 3 November 2016. Retrieved 25 March 2021.
- ↑ Salahudeen MJ; Duffull SB; Nishtala PS; et al. (2015-03-25). "Anticholinergic burden quantified by anticholinergic risk scales and adverse outcomes in older people: a systematic review". BMC Geriatrics. 15 (31): 31. doi:10.1186/s12877-015-0029-9. PMC 4377853. PMID 25879993.
- ↑ L.A. Walter, U.S. Patent 3,061,517 (1962)
- ↑ L.A. Walter, U.S. Patent 3,030,371 (1962)
- ↑ Barondes, Samuel H. (2003). Better Than Prozac. New York: Oxford University Press. pp. 39–40. ISBN 0-19-515130-5.
External links
| External sites: | |
|---|---|
| Identifiers: |
|
- Pages using duplicate arguments in template calls
- CS1 maint: date format
- Drugs with non-standard legal status
- Chemical articles with unknown parameter in Infobox drug
- Chemical articles without CAS registry number
- Articles without EBI source
- Chemical pages without ChemSpiderID
- Chemical pages without DrugBank identifier
- Articles without KEGG source
- Articles without UNII source
- Drugs missing an ATC code
- Drugboxes which contain changes to watched fields
- All articles with unsourced statements
- Articles with unsourced statements from August 2018
- Articles with invalid date parameter in template
- All articles with specifically marked weasel-worded phrases
- Articles with specifically marked weasel-worded phrases from September 2020
- Articles with unsourced statements
- Pfizer brands
- Wyeth brands
- H1 receptor antagonists
- Muscarinic antagonists
- Bromoarenes
- Pyridines
- Serotonin-norepinephrine reuptake inhibitors
- RTT