Meclizine
 | |
| Names | |
|---|---|
| Trade names | Bonine, Antivert, others |
| Other names | Meclozine |
| |
| Clinical data | |
| Drug class | Antihistamine[1] |
| Main uses | Motion sickness, vertigo[1] |
| Side effects | Sleepiness, dry mouth[1] |
| Pregnancy category |
|
| Routes of use | By mouth, under the tongue, in the cheek |
| Defined daily dose | 50 mg[2] |
| External links | |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a682548 |
| Legal | |
| License data | |
| Legal status | |
| Pharmacokinetics | |
| Metabolism | Liver |
| Elimination half-life | 6 hours |
| Chemical and physical data | |
| Formula | C25H27ClN2 |
| Molar mass | 390.96 g·mol−1 |
| 3D model (JSmol) | |
| Boiling point | 230 °C (446 °F) |
| |
| |
Meclizine, sold under the brand names Bonine among others, is an antihistamine used to treat motion sickness and the feeling like the world is spinning (vertigo).[1] It is taken by mouth.[1] Effects generally begin in an hour and last for up to a day.[1]
Common side effects include sleepiness and dry mouth.[1] Serious side effects may include allergic reactions.[1] Use in pregnancy appears safe but has not been well studied while use in breastfeeding is of unclear safety.[3] It is believed to work in part by anticholinergic and antihistamine mechanisms.[1]
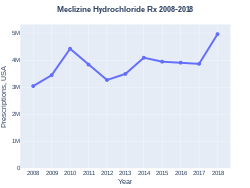
Meclizine was patented in 1951 and came into medical use in 1953.[4] It is available as a generic medication and often over the counter.[1][5] In the United States the wholesale cost per dose is about US$0.03.[6] It is not available in Australia.[7] In 2017, it was the 161st most commonly prescribed medication in the United States, with more than three million prescriptions.[8][9]
Medical uses
Meclizine is used to treat symptoms of motion sickness. Safety and efficacy in children younger than twelve years of age has not been established; therefore, use in this population is not recommended.[citation needed] Meclizine should be taken with caution in the elderly due to increased risk of confusion and amnesia.[10]
Motion sickness
Meclizine is effective in inhibiting nausea, vomiting, and dizziness caused by motion sickness.[11]
The drug is safe for treating nausea in pregnancy and is a first-line therapy for this use.[12][13] Doxylamine is similarly safe. Meclizine may not be strong enough for especially sickening motion stimuli and second-line defenses should be tried in those cases.[14]
Vertigo
Meclizine may be used to treat motion sickness or vertigo such as in those with Meniere's disease.[15][16]
Dosage
The defined daily dose is 50 mg by mouth or rectally.[2]
Side effects
Some common side effects such as drowsiness, dry mouth, and tiredness may occur. Meclizine has been shown to have fewer dry mouth side effects than the traditional treatment for motion sickness, transdermal scopolamine.[17] A very serious allergic reaction to this drug is unlikely, but immediate medical attention should be sought if it occurs. Symptoms of a serious allergic reaction may include rash, itching, swelling, severe dizziness, and trouble breathing.[18]
Drowsiness
Drowsiness may result as a side effect of taking meclizine. Users are advised not to operate heavy machinery while under the influence. The consumption of alcohol while under the influence of meclizine may result in additional drowsiness.
Elderly
As with any anticholinergic agent, meclizine may cause confusion or aggravate symptoms in those with dementia in the geriatric population (older than 65 years). Therefore, caution should be used when administering meclizine to the elderly.[19]
Mechanism of action
Meclizine is an antagonist at H1 receptors. It possesses anticholinergic, central nervous system depressant, and local anesthetic effects. Its antiemetic and antivertigo effects are not fully understood, but its central anticholinergic properties are partially responsible. The drug depresses labyrinth excitability and vestibular stimulation, and it may affect the medullary chemoreceptor trigger zone.[20] It has however been suggested that meclizine only has an inhibitory effect under normal viewing-circumstances, as the drug has been shown to enhance an isolated vestibular response. Much like motion-sickness arises from a discrepancy between multiple senses, Meclizine most likely affects a wide array of sensory mechanisms related to self-motion.[21] Meclizine also is a dopamine antagonist at D1-like and D2-like receptors[citation needed] but does not cause catalepsy[note 1] in mice, perhaps because of its anticholinergic activity.[22]
Chemistry
Meclizine is a first-generation antihistamine (nonselective H1 antagonist) of the piperazine class. It is structurally and pharmacologically similar to buclizine, cyclizine, and hydroxyzine, but has a shorter half-life of six hours compared to cyclizine and hydroxyzine with about 20 hours (though half-life should not be confused with duration). It is used as an antivertigo/antiemetic agent, specifically in the prevention and treatment of nausea, vomiting, and dizziness associated with motion sickness.[20] Meclizine is sometimes combined with opioids, especially ones of the open-chain class like methadone, dextropropoxyphene, and dipipanone. Similarly, Diconal is a combination drug containing dipipanone and cyclizine.[citation needed]
Synthesis
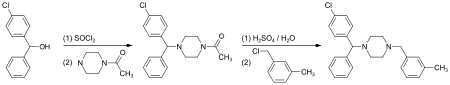
(4-Chlorphenyl)-phenylmethanol is halogenated with thionyl chloride before adding acetylpiperazine. The acetyl group is cleaved with diluted sulfuric acid. An N-alkylation of the piperazine ring with 3-methylbenzylchloride completes the synthesis.[23]
Alternatively, the last step can be replaced by a reductive N-alkylation with 3-methylbenzaldehyde. The reductive agent is hydrogen, and Raney nickel is used as a catalyst.[24][25]
Meclizine is obtained and used as a racemate, a 1:1 mixture of the two stereoisomers. Drug forms contain the racemic dihydrochloride.
Names
Meclizine is an international nonproprietary name.[26]
It is sold under the brand names Bonine, Bonamine, Antivert, Postafen, Sea Legs, and Dramamine II (Less Drowsy Formulation). Emesafene is a combination of meclizine (1/3) and pyridoxine (2/3). In Canada, Antivert Tab (which is no longer available) was a combination of meclizine and nicotinic acid.[27]
Society and culture
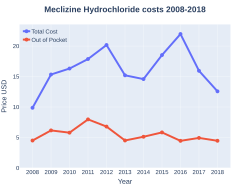
Cost
In the United States the wholesale cost per dose is about US$0.03.[6] In 2017, it was the 161st most commonly prescribed medication in the United States, with more than three million prescriptions.[8][9]
-
Meclizine costs (US)
-
Meclizine prescriptions (US)
Notes
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 1.6 1.7 1.8 1.9 "Meclizine Hydrochloride Monograph for Professionals". Drugs.com. American Society of Health-System Pharmacists. Archived from the original on 24 December 2019. Retrieved 22 March 2019.
- ↑ 2.0 2.1 "WHOCC - ATC/DDD Index". www.whocc.no. Archived from the original on 1 November 2020. Retrieved 7 September 2020.
- ↑ "Meclizine Use During Pregnancy". Drugs.com. Archived from the original on 1 December 2020. Retrieved 3 March 2019.
- ↑ Fischer, Jnos; Ganellin, C. Robin (2006). Analogue-based Drug Discovery. John Wiley & Sons. p. 547. ISBN 9783527607495. Archived from the original on 6 March 2019. Retrieved 3 March 2019.
- ↑ Cappa M, Cianfarani S, Ghizzoni L, Loche S, Maghnie M (2015). Advanced Therapies in Pediatric Endocrinology and Diabetology: Workshop, Rome, October 2014. Karger Medical and Scientific Publishers. p. 101. ISBN 9783318056372. Archived from the original on 23 December 2019. Retrieved 22 March 2019.
- ↑ 6.0 6.1 "NADAC as of 2019-02-27". Centers for Medicare and Medicaid Services. Archived from the original on 6 March 2019. Retrieved 3 March 2019.
- ↑ McKenzie, Gayle; Broyles, Bonita; Evans, Mary E.; Page, Rachel; Pleunik, Sussan; Reiss, Barry S. (2016). Pharmacology in Nursing: Australian and New Zealand Edition with Student Resource Access 12 Months. Cengage AU. p. 569. ISBN 9780170362030. Archived from the original on 19 December 2019. Retrieved 22 March 2019.
- ↑ 8.0 8.1 "The Top 300 of 2020". ClinCalc. Archived from the original on 12 February 2021. Retrieved 11 April 2020.
- ↑ 9.0 9.1 "Meclizine Hydrochloride - Drug Usage Statistics". ClinCalc. Archived from the original on 26 February 2021. Retrieved 11 April 2020.
- ↑ MICROMEDEX 2.0 Archived 21 June 2019 at the Wayback Machine. Accessed November 7, 2010.[full citation needed]
- ↑ "Drugs & Medications". www.webmd.com. Archived from the original on 29 December 2018. Retrieved 28 December 2018.
- ↑ "Antiemetische Therapie bei Schwangerschaftserbrechen" [Antiemetic therapy in pregnancy]. Arznei-Telegramm (in German). 40: 87–89. 2009. Archived from the original on 22 October 2010. Retrieved 19 December 2010.
{{cite journal}}: CS1 maint: unrecognized language (link) - ↑ Embryotox: Meclozin Archived 23 October 2010 at the Wayback Machine (in German)
- ↑ Lawson BD, McGee HA, Castaneda MA, Golding JF, Kass SJ, McGrath CM (2 December 2009). "Evaluation of several common antimotion sickness medications and recommendations concerning their potential usefulness during special operations". Pensacola, Florida: Naval Aerospace Research Lab. Archived from the original on 27 April 2016. Retrieved 7 February 2016.
- ↑ Nakashima, T; Pyykkö, I; Arroll, MA; Casselbrant, ML; Foster, CA; Manzoor, NF; Megerian, CA; Naganawa, S; Young, YH (12 May 2016). "Meniere's disease". Nature Reviews. Disease Primers. 2: 16028. doi:10.1038/nrdp.2016.28. PMID 27170253.
- ↑ "Meclizine". LiverTox. 2017. PMID 31643231. Archived from the original on 28 August 2021. Retrieved 12 January 2020.
- ↑ Dahl E, Offer-Ohlsen D, Lillevold PE, Sandvik L (July 1984). "Transdermal scopolamine, oral meclizine, and placebo in motion sickness". Clinical Pharmacology and Therapeutics. 36 (1): 116–20. doi:10.1038/clpt.1984.148. PMID 6734040.
- ↑ Meclizine - oral, Antivert, D-vert, Dramamine II Archived 10 November 2010 at the Wayback Machine. Accessed November 7, 2010.
- ↑ Merck Manuals, Online Medical Library: Meclizine (Drug Information Provided by Lexi-Comp) Archived 21 September 2009 at the Wayback Machine, revised January 2010, accessed November 7, 2010.
- ↑ 20.0 20.1 Clinical Pharmacology. Clinical Pharmacology Archived 23 April 2021 at the Wayback Machine, revised November 20, 2009, accessed November 7, 2010.[full citation needed]
- ↑ Wibble, T; Engström, J; Verrecchia, L; Pansell, T. "The effects of meclizine on motion sickness revisited". Br J Clin Pharmacol. doi:10.1111/bcp.14257.
- ↑ 22.0 22.1 Haraguchi K, Ito K, Kotaki H, Sawada Y, Iga T (June 1997). "Prediction of drug-induced catalepsy based on dopamine D1, D2, and muscarinic acetylcholine receptor occupancies". Drug Metabolism and Disposition. 25 (6): 675–84. PMID 9193868. Archived from the original on 28 August 2021. Retrieved 12 June 2014.
- ↑ Fuhrkop JH, Li G (2003). Organic Synthesis. Concepts and Methods. Wiley. p. 237. ISBN 978-3-527-30272-7.
- ↑ US 2 709 169 (UCB, 1955)
- ↑ Kleemann A, Engel J, Kutscher B, Reichert D (2001). Pharmaceutical Substances. Synthesis, Patents, Applications (4th ed.). Thieme. ISBN 3-13-115134-X.
- ↑ Guidelines on the Use of INNs for Pharmaceutical Substances (1997). Archived 18 October 2012 at the Wayback Machine Accessed November, 2013 "Guidance on INN." WHO.
- ↑ DrugBank. Drugbank: Drug card for Meclizine Archived 29 October 2010 at the Wayback Machine David Wishard: University of Alberta, Canada. Accessed November 7, 2010.
External links
| Identifiers: |
|
|---|
- "Meclizine". Drug Information Portal. U.S. National Library of Medicine. Archived from the original on 13 January 2020. Retrieved 13 January 2020.
- Pages using duplicate arguments in template calls
- Webarchive template wayback links
- All articles with incomplete citations
- Articles with incomplete citations from August 2010
- Articles with invalid date parameter in template
- CS1 maint: unrecognized language
- Articles with German-language sources (de)
- Articles with hatnote templates targeting a nonexistent page
- Use dmy dates from January 2020
- Chemical articles with unknown parameter in Infobox drug
- Chemical articles without CAS registry number
- Articles without EBI source
- Chemical pages without ChemSpiderID
- Chemical pages without DrugBank identifier
- Articles without KEGG source
- Articles without UNII source
- Drugs missing an ATC code
- Drugboxes which contain changes to verified fields
- Drugboxes which contain changes to watched fields
- All articles with unsourced statements
- Articles with unsourced statements from March 2019
- Articles with unsourced statements from September 2015
- Articles with changed DrugBank identifier
- Articles with changed EBI identifier
- Antiemetics
- Chlorcyclizines
- Motion sickness
- Pregnane X receptor agonists
- RTT