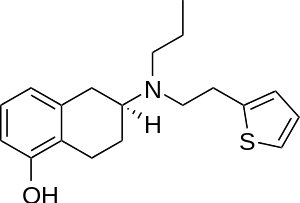
Rotigotine
 | |
| Names | |
|---|---|
| Trade names | Neupro, Leganto |
| |
| Clinical data | |
| Drug class | Dopamine agonist[1] |
| Main uses | Parkinson's (PD), restless legs syndrome (RLS)[1] |
| Side effects | Nausea, sleepiness, increased sweating, anxiety, swelling[1] |
| Pregnancy category |
|
| Routes of use | Transdermal patch |
| Typical dose | 1 to 8 mg/24 hours[1] |
| External links | |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a607059 |
| Legal | |
| License data |
|
| Legal status | |
| Pharmacokinetics | |
| Bioavailability | 37% (transdermal) |
| Protein binding | 92% |
| Metabolism | Liver (CYP-mediated) |
| Elimination half-life | 5–7 hours |
| Excretion | Urine (71%), Fecal (23%) |
| Chemical and physical data | |
| Formula | C19H25NOS |
| Molar mass | 315.48 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Rotigotine, sold under the brand name Neupro among others, used to treat Parkinson's disease (PD) and restless legs syndrome (RLS).[1] It comes as a once-daily skin patch.[1]
Common side effects include nausea, sleepiness, increased sweating, anxiety, and swelling.[1] Other side effects may include anaphylaxis, sleep attacks, psychosis, compulsive gambling, and weight gain.[1] There are concerns regarding safety in pregnancy.[2] It is a dopamine agonist of the non-ergoline class.[1]
Rotigotine was approved for medical use in Europe in 2006 and the United States in 2007.[3][1] In the United States it costs about 750 USD per month as of 2021.[4] In the United Kingdom this amount costs the NHS about £130.[5]
Medical uses
Dosage
It is used at a dose of 1 to 8 mg/24 hours.[1]
Side effects
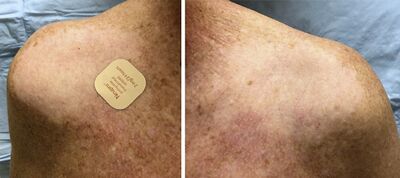
General side effects for rotigotine may include constipation, dyskinesia, nausea, vomiting, dizziness, fatigue, insomnia, somnolence, confusion, and hallucinations.[6][7] More serious complications can include psychosis and impulse control disorders like hypersexuality, punding, and pathological gambling.[8] Mild adverse skin reactions at the patch application site may also occur.[9][7]
Pharmacology
Rotigotine acts as a non-selective agonist of the dopamine D1, D2, D3, and, to a lesser extent, D4 and D5 receptors, with highest affinity for the D3 receptor.[10] In terms of affinity, rotigotine has 10-fold selectivity for the D3 receptor over the D2, D4, and D5 receptors and 100-fold selectivity for the D3 receptor over the D1 receptor.[10] In functional studies however, rotigotine behaves as a full agonist of D1, D2, and D3 with similar potencies (EC50).[10] Its ability to activate both D1-like and D2-like receptors is similar to the case of apomorphine (which notably has greater efficacy in the treatment of Parkinson's disease than D2-like-selective agonists but has suboptimal pharmacokinetic properties) and pergolide but unlike pramipexole and ropinirole.[10]
Rotigotine possesses the following in vitro receptor binding profile:[11]
|
|
|
All affinities listed were assayed using human materials except that for α2B-adrenergic which was done with NG 108–15 cells. Rotigotine behaves as a partial or full agonist (depending on the assay) at all dopamine receptors listed, as an antagonist at the α2B-adrenergic receptor, and as a partial agonist at the 5-HT1A receptor.[11] Though it has affinity for a large number of sites as shown above, at clinical doses rotigotine behaves mostly as a selective D1-like (D1, D5) and D2-like (D2, D3, D4) receptor agonist, with its α2B-adrenergic and 5-HT1A activity also possibly having some minor relevance.
History
Rotigotine was first developed in 1985 as N-0437 by a team from the University of Groningen.[12] Development was then continued by Aderis Pharmaceuticals. In 1998, Aderis licensed worldwide development and commercialization rights for rotigotine to the German pharmaceutical company Schwarz Pharma (today a subsidiary of the Belgian company UCB S.A.).[13]
The drug was approved by the European Medicines Agency (EMA) for use in Europe in 2006.[14] In 2007, the Neupro patch was approved by the Food and Drug Administration (FDA) as the first transdermal treatment of Parkinson's disease in the United States. In 2008, Schwarz Pharma recalled all Neupro patches in the United States and some in Europe because of problems with the delivery mechanism. The patch was reformulated, and was reintroduced in the United States in 2012.[15]
Rotigotine was authorized as a treatment for restless legs syndrome in August 2008.[16]
See also
References
- ↑ 1.00 1.01 1.02 1.03 1.04 1.05 1.06 1.07 1.08 1.09 1.10 "Rotigotine Monograph for Professionals". Drugs.com. Archived from the original on 2 May 2021. Retrieved 19 October 2021.
- ↑ "Rotigotine (Neupro) Use During Pregnancy". Drugs.com. Archived from the original on 27 October 2020. Retrieved 19 October 2021.
- ↑ "Neupro". Archived from the original on 2 March 2020. Retrieved 19 October 2021.
- ↑ "Neupro Prices, Coupons & Patient Assistance Programs". Drugs.com. Archived from the original on 24 January 2021. Retrieved 19 October 2021.
- ↑ BNF (80 ed.). BMJ Group and the Pharmaceutical Press. September 2020 – March 2021. p. 446. ISBN 978-0-85711-369-6.
- ↑ Kulisevsky J, Pagonabarraga J (2010). "Tolerability and safety of ropinirole versus other dopamine agonists and levodopa in the treatment of Parkinson's disease: meta-analysis of randomized controlled trials". Drug Safety. 33 (2): 147–61. doi:10.2165/11319860-000000000-00000. PMID 20082541. S2CID 34876593.
- ↑ 7.0 7.1 Parkinson Study Group (December 2003). "A controlled trial of rotigotine monotherapy in early Parkinson's disease". Archives of Neurology. 60 (12): 1721–8. doi:10.1001/archneur.60.12.1721. PMID 14676046.
- ↑ Wingo TS, Evatt M, Scott B, Freeman A, Stacy M (2009). "Impulse control disorders arising in 3 patients treated with rotigotine". Clinical Neuropharmacology. 32 (2): 59–62. doi:10.1097/WNF.0B013E3181684542. PMID 18978496. S2CID 23942499.
- ↑ Chen JJ, Swope DM, Dashtipour K, Lyons KE (December 2009). "Transdermal rotigotine: a clinically innovative dopamine-receptor agonist for the management of Parkinson's disease". Pharmacotherapy. 29 (12): 1452–67. doi:10.1592/phco.29.12.1452. PMID 19947805. S2CID 40466260.
- ↑ 10.0 10.1 10.2 10.3 Wood, Martyn; Dubois, Vanessa; Scheller, Dieter; Gillard, Michel (2015). "Rotigotine is a potent agonist at dopamine D1receptors as well as at dopamine D2and D3receptors". British Journal of Pharmacology. 172 (4): 1124–1135. doi:10.1111/bph.12988. ISSN 0007-1188. PMC 4314200. PMID 25339241.
- ↑ 11.0 11.1 Scheller D, Ullmer C, Berkels R, Gwarek M, Lübbert H (January 2009). "The in vitro receptor profile of rotigotine: a new agent for the treatment of Parkinson's disease". Naunyn-Schmiedeberg's Archives of Pharmacology. 379 (1): 73–86. doi:10.1007/s00210-008-0341-4. PMID 18704368. S2CID 25112443.
- ↑ Horn AS, Tepper P, Van der Weide J, Watanabe M, Grigoriadis D, Seeman P (1985). "Synthesis and radioreceptor binding activity of N-0437, a new, extremely potent and selective D2 dopamine receptor agonist". Pharm Weekbl Sci. 7 (5): 208–11. doi:10.1007/bf02307578. PMID 2933633. S2CID 8847550.
- ↑ Development & Commercialization of rotigotine by Aderis (Aderis Pharmaceuticals making a reference for the commercialization of rotigotine Archived 3 September 2011 at the Wayback Machine)
- ↑ "Neupro EPAR". European Medicines Agency. 17 September 2018. Archived from the original on 2 March 2020. Retrieved 2 March 2020.
- ↑ "Neupro Patch Re-launches in the US". Archived from the original on 23 March 2016. Retrieved 27 March 2021.
- ↑ Davies S (September 2009). "Rotigotine for restless legs syndrome". Drugs of Today. 45 (9): 663–8. doi:10.1358/dot.2009.45.9.1399952. PMID 19956807. Archived from the original on 16 January 2020. Retrieved 27 March 2021.
External links
| External sites: | |
|---|---|
| Identifiers: |
|
- Pages using duplicate arguments in template calls
- Webarchive template wayback links
- Use dmy dates from March 2020
- Articles with invalid date parameter in template
- Drugs with non-standard legal status
- Chemical articles with unknown parameter in Infobox drug
- Chemical articles without CAS registry number
- Articles without EBI source
- Chemical pages without ChemSpiderID
- Chemical pages without DrugBank identifier
- Articles without KEGG source
- Articles without UNII source
- Drugs missing an ATC code
- Drug has EMA link
- Drugboxes which contain changes to verified fields
- Articles with changed CASNo identifier
- Articles with changed EBI identifier
- Aminotetralins
- Dopamine agonists
- Phenols
- Thiophenes
- RTT