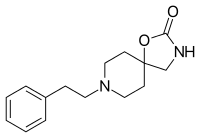
Fenspiride
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Eurespal, Pneumorel |
| AHFS/Drugs.com | International Drug Names |
| Routes of administration | Oral |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 90%[1] |
| Elimination half-life | 14–16 hours |
| Excretion | Urine (90%), feces (~10%) |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.023.411 |
| Chemical and physical data | |
| Formula | C15H20N2O2 |
| Molar mass | 260.337 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Fenspiride (INN, brand names Eurespal, Pneumorel and others) is an oxazolidinone spiro compound used as a drug in the treatment of certain respiratory diseases.[2] The pharmacotherapeutic classification is antitussives. In Russia it was approved for the treatment of acute and chronic inflammatory diseases of ENT organs (ear, nose, throat) and the respiratory tract (like rhinopharyngitis, laryngitis, tracheobronchitis, otitis and sinusitis), as well as for maintenance treatment of asthma.[3] Russia, Romania, France and other European countries withdrew fenspiride-based drugs from the market due to the risk of QT prolongation and torsades de pointes.[4] Fenspiride is known to have activity as an alpha-1 blocker, H1 antagonist, it also inhibits PDE3, PDE4, PDE5 with -logIC50 values of 3.44, 4.16, 3.8 respectively.[5]
References
- ^ Montes B, Catalan M, Roces A, Jeanniot JP, Honorato JM (1993). "Single dose pharmacokinetics of fenspiride hydrochloride: phase I clinical trial". European Journal of Clinical Pharmacology. 45 (2): 169–72. doi:10.1007/bf00315501. PMID 7901024. S2CID 20605939.
- ^ Płusa T, Nawacka D (December 1998). "[Efficacy and tolerance of fenspiride in adult patients with acute respiratory tract infections]". Polski Merkuriusz Lekarski. 5 (30): 368–71. PMID 10101527.
- ^ "Эреспал (Eurespal) Prescribing Information. VIDAL Drug Compendium" (in Russian). Retrieved 6 February 2014.
- ^ "Fenspiride containing medicinal products". European Medicines Agency. 2019-02-15. Retrieved 2019-06-12.
- ^ Jankowski R (September 2002). "[ENT inflammation and importance of fenspiride]". Presse Med (in French). 31 Spec No 1: HS7–10. PMID 12378970.
- CS1 Russian-language sources (ru)
- CS1: long volume value
- CS1 French-language sources (fr)
- Articles with short description
- Short description matches Wikidata
- Drugs with non-standard legal status
- Articles with changed DrugBank identifier
- Articles with changed ChemSpider identifier
- Articles with changed EBI identifier
- ECHA InfoCard ID from Wikidata
- Articles with changed InChI identifier
- Drugboxes which contain changes to verified fields
- Drugboxes which contain changes to watched fields
- 2-Oxazolidinones
- Alpha-1 blockers
- Bronchodilators
- Phenethylamines
- Spiro compounds
- Withdrawn drugs
- All stub articles
- Respiratory system drug stubs