Metoprolol
 | |
| Names | |
|---|---|
| Pronunciation | /mɛˈtoʊproʊlɑːl/, /mɛtoʊˈproʊlɑːl/ |
| Trade names | Lopressor, Metolar XR, Toprol XL, others |
| |
| Clinical data | |
| Drug class | Beta blocker (β1 selective) |
| Main uses | High blood pressure, fast heart beat, angina[1] |
| Side effects | Trouble sleeping, feeling tired, feeling faint[1] |
| Pregnancy category |
|
| Routes of use | By mouth, IV |
| Defined daily dose | 150 mg (by mouth or injection)[2] |
| External links | |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a682864 |
| Legal | |
| License data |
|
| Legal status | |
| Pharmacokinetics | |
| Bioavailability | 50% (single dose)[3] 70% (repeated administration)[4] |
| Protein binding | 12% |
| Metabolism | Liver via CYP2D6, CYP3A4 |
| Elimination half-life | 3–7 hours[5] |
| Excretion | Kidney |
| Chemical and physical data | |
| Formula | C15H25NO3 |
| Molar mass | 267.369 g·mol−1 |
| 3D model (JSmol) | |
| Chirality | Racemic mixture |
| Melting point | 120 °C (248 °F) |
| |
| |
Metoprolol, marketed under the tradename Lopressor among others, is a medication of the beta blocker type.[1] It is used to treat high blood pressure, chest pain due to poor blood flow to the heart, and a number of conditions involving an abnormally fast heart rate.[1] It is also used to prevent further heart problems after myocardial infarction and to prevent headaches in those with migraines.[1]
Metoprolol is sold in formulations that can be taken by mouth or given intravenously.[1] The medication is often taken twice a day.[1] The extended-release formulation is taken once per day.[1] Metoprolol may be combined with hydrochlorothiazide (a diuretic) in a single tablet.[1]
Common side effects include trouble sleeping, feeling tired, feeling faint, and abdominal discomfort.[1] Large doses may cause serious toxicity.[6][7] Risk in pregnancy has not been ruled out.[1][8] It appears to be safe in breastfeeding.[9] Greater care is required with use in those with liver problems or asthma.[1] Stopping this drug should be done slowly to decrease the risk of further health problems.[1]
Metoprolol was first made in 1969, patented in 1970, and approved for medical use in 1982.[10][11] It is on the World Health Organization's List of Essential Medicines as an alternative to bisoprolol.[12] It is available as a generic medication.[1] The cost in the developing world as of 2015 was about 0.04 USD per 100 mg dose.[13] In 2017, it was the sixth most commonly prescribed medication in the United States, with more than 68 million prescriptions.[14][15]
Medical uses
Metoprolol is used for a number of conditions, including hypertension, angina, acute myocardial infarction, supraventricular tachycardia, ventricular tachycardia, congestive heart failure, and prevention of migraine headaches.[1]
- Adjunct in treatment of hyperthyroidism[16]
The different salt versions of metoprolol, metoprolol tartrate and metoprolol succinate, are approved for different conditions and are not interchangeable.[17][18][19]
Off-label uses include supraventricular tachycardia and thyroid storm.[20]
Available forms
Metoprolol is sold in formulations that can be taken by mouth or given intravenously.[1] The medication is often taken twice a day.[1] The extended-release formulation is taken once per day.[1] Metoprolol may be combined with hydrochlorothiazide (a diuretic) in a single tablet.[1]
Dosage
The defined daily dose for metoprolol is 150 mg by injection or by mouth.[2] The typical dose by mouth; however, can range from 25 mg to 400 mg and is taken either once or twice per day depending on the formulation.[1]
For rapid atrial fibrillation 5 mg every two minutes by injection into a vein up to three doses maybe used.[1] The person may than be started on 25 to 50 mg of metoprolol 15 minutes after the last injection.[1]
Side effects
Side effects, especially with higher doses, include dizziness, drowsiness, fatigue, diarrhea, unusual dreams, trouble sleeping, depression, and vision problems. β-blockers, including metoprolol, reduce salivary flow via inhibition of the direct sympathetic innervation of the salivary glands.[21][22] Metoprolol may also cause the hands and feet to feel cold.[23] Due to the high penetration across the blood-brain barrier, lipophilic beta blockers such as propranolol and metoprolol are more likely than other less lipophilic beta blockers to cause sleep disturbances such as insomnia, vivid dreams and nightmares.[24]
Serious side effects that are advised to be reported immediately include symptoms of bradycardia (resting heart rate slower than 60 beats per minute), persistent symptoms of dizziness, fainting and unusual fatigue, bluish discoloration of the fingers and toes, numbness/tingling/swelling of the hands or feet, sexual dysfunction, erectile dysfunction, hair loss, mental/mood changes, depression, breathing difficulty, cough, dyslipidemia and increased thirst. Consuming alcohol while taking metoprolol may cause mild body rashes and is not advised.[20]
Precautions
Metoprolol may worsen the symptoms of heart failure in some patients, who may experience chest pain or discomfort, dilated neck veins, extreme fatigue, irregular breathing, an irregular heartbeat, shortness of breath, swelling of the face, fingers, feet, or lower legs, weight gain, or wheezing.[25]
This medicine may cause changes in blood sugar levels or cover up signs of low blood sugar, such as a rapid pulse rate.[25] It also may cause some people to become less alert than they are normally, making it dangerous for them to drive or use machines.[25]
Greater care is required with use in those with liver problems or asthma.[1] Stopping this drug should be done slowly to decrease the risk of further health problems.[1]
Pregnancy and breastfeeding
Risk for the fetus has not been ruled out, per being rated pregnancy category C in the United States.[1][8] Metoprolol is category C in Australia, meaning that it may be suspected of causing harmful effects on the human fetus (but no malformations).[8] It appears to be safe in breastfeeding.[9]
Overdose
Excessive doses of metoprolol can cause severe hypotension, bradycardia, metabolic acidosis, seizures, and cardiorespiratory arrest. Blood or plasma concentrations may be measured to confirm a diagnosis of overdose or poisoning in hospitalized patients or to assist in a medicolegal death investigation. Plasma levels are usually less than 200 μg/l during therapeutic administration, but can range from 1–20 mg/l in overdose.[26][27][28]
Pharmacology
General pharmacological principles of metoprolol:[medical citation needed]
- beta-1 selective
- moderately lipophilic
- without intrinsic sympathomimetic activity
- with weak membrane stabilizing activity
- decreases heart rate, contractility, and cardiac output, therefore decreasing blood pressure
Mechanism of action
Metoprolol blocks β1 adrenergic receptors in heart muscle cells, thereby decreasing the slope of phase 4 in the nodal action potential (reducing Na+ uptake) and prolonging repolarization of phase 3 (slowing down K+ release).[29] It also suppresses the norepinephrine-induced increase in the sarcoplasmic reticulum (SR) Ca2+ leak and the spontaneous SR Ca2+ release, which are the major triggers for atrial fibrillation.[29]
Pharmacokinetics
It undergoes α-hydroxylation and O-demethylation as a substrate of the cytochrome liver enzymes CYP2D6.[30][31]
Chemistry
The active substance metoprolol is employed either as metoprolol succinate or as metoprolol tartrate (where 100 mg metoprolol tartrate corresponds to 95 mg metoprolol succinate). The tartrate is an immediate-release formulation and the succinate is an extended-release formulation.[32]
Stereochemistry
Metoprolol contains a stereocenter and consists of two enantiomers. This is a racemate, i.e. a 1:1 mixture of (R)- and the (S)-form:[33]
| Enantiomers of metoprolol | |
|---|---|
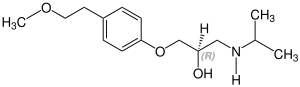 CAS-Nummer: 81024-43-3 |
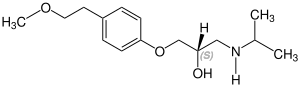 CAS-Nummer: 81024-42-2 |
History
Metoprolol was first discovered in 1969 by Bengt Ablad and Enar Carlsson.[10]
Society and culture
Within the UK, metoprolol is classified as a prescription-only drug in the beta blocker class and is regulated by the Medicines and Healthcare Products Regulatory Agency (MHRA). The MHRA is a government body set up in 2003 and is responsible for regulating medicines, medical devices, and equipment used in healthcare. The MHRA acknowledges that no product is completely risk free but takes into account research and evidence to ensure that any risks associated are minimal.[34]
The use of beta blockers such as metoprolol was approved in the U.S. by the Food and Drug Administration (FDA) in 1967. The FDA has approved beta blockers for the treatment of cardiac arrhythmias, hypertension, migraines, and others. Prescribers may choose to prescribe beta blockers for other treatments if there is just cause even though it is not approved by the FDA. Drug manufacturers, however, are unable to advertise beta blockers for other purposes that have not been approved by the FDA. Since the FDA does not regulate the practice of medicine after the drug has been approved, it is legal to prescribe beta blockers for other treatments such as performance anxiety.[35]
Cost
The cost in the developing world as of 2015 was about 0.04 USD per 100 mg dose.[13] In 2017, it was the sixth most commonly prescribed medication in the United States, with more than 68 million prescriptions.[14][15]
-
Metoprolol costs (US)
-
Metoprolol prescriptions (US)
Legislation
On 23 September 2011, the Medicines and Healthcare products Regulatory Agency (MHRA) granted the Intas Pharmaceuticals Limited marketing authorization (licences) for metoprolol tartrate (50 mg and 100 mg tablets) for medicinal prescription only; this was after it was established that there were no new or unexpected safety concerns and that the benefits of metoprolol tartrate were greater than the risks.[36] Metoprolol tartrate is a generic name of Lopressor, which was licensed and authorized on 6 June 1997 to Novartis Pharmaceuticals.[37]
Sport
Metoprolol, due to being a beta blocker, is banned by the world anti-doping agency in some sports. Beta blockers can be used to reduce heart rate and minimize tremors, which can enhance performance in sports such as archery.[38] All beta blockers are banned during and out of competition for archery and shooting.[39] In some sports such as all forms of billiards, darts, and golf, beta blockers are banned during competition only. Furthermore, any form of beta blocker is banned within riflery competitions by the National Collegiate Athletic Association.[40]
To detect if beta blockers have been used trace analysis of human urine is analyzed. Uncharged drugs and/or metabolites of beta blockers can be analysed by gas chromatography-mass spectrometry in selected ion monitoring (GC-MS-SIM). However, in modern times it is increasingly difficult to detect the presence of beta blockers used for sports doping purposes. A disadvantage to using GC-MS-SIM is that prior knowledge of the molecular structure of the target drugs/metabolites is required. Modern times have shown a variance in structures and hence novel beta blockers can go undetected.[41]
Lawsuit
In 2012, an $11 million settlement was reached with Toprol XL (time-release formula version of metoprolol) and its generic equivalent metoprolol. The lawsuit involved the pharmaceuticals companies AstraZeneca AB, AstraZeneca LP, AstraZeneca Pharmaceuticals LP, and Aktiebolaget Hassle. The claims of the lawsuit advise that the manufacturers violated antitrust and consumer protection law. Claiming that to increase profits, lower cost generic versions of Toprol XL were intentionally kept off the market. This claim was subsequently denied by the defendants.[42]
References
- ↑ 1.00 1.01 1.02 1.03 1.04 1.05 1.06 1.07 1.08 1.09 1.10 1.11 1.12 1.13 1.14 1.15 1.16 1.17 1.18 1.19 1.20 1.21 1.22 1.23 1.24 "Metoprolol". The American Society of Health-System Pharmacists. Archived from the original on 2014-03-12. Retrieved 21 April 2014.
- ↑ 2.0 2.1 "WHOCC - ATC/DDD Index". www.whocc.no. Archived from the original on 20 September 2020. Retrieved 22 September 2020.
- ↑ "Metolar 25/50 (metoprolol tartrate) tablet" (PDF). Food and Drug Administration (FDA). Archived (PDF) from the original on 3 March 2016. Retrieved 5 May 2015.
- ↑ Jasek, W, ed. (2007). Austria-Codex (in German) (62nd ed.). Vienna: Österreichischer Apothekerverlag. pp. 916–919. ISBN 978-3-85200-181-4.
{{cite book}}: CS1 maint: unrecognized language (link) - ↑ Benowitz, Neal L. (2020). "11. Antihypertensive agents". In Katzung, Bertram G.; Trevor, Anthony J. (eds.). Basic and Clinical Pharmacology (15th ed.). New York: McGraw-Hill. p. 183. ISBN 978-1-260-45231-0. Archived from the original on 10 October 2021. Retrieved 5 December 2021.
- ↑ Pillay (2012). Modern Medical Toxicology. Jaypee Brothers Publishers. p. 303. ISBN 9789350259658. Archived from the original on 2017-07-07.
- ↑ Marx, John A. Marx (2014). "Cardiovascular Drugs". Rosen's emergency medicine : concepts and clinical practice (8th ed.). Philadelphia, PA: Elsevier/Saunders. pp. Chapter 152. ISBN 978-1455706051.
- ↑ 8.0 8.1 8.2 "Prescribing medicines in pregnancy database". Australian Government. 3 March 2014. Archived from the original on 8 April 2014. Retrieved 22 April 2014.
- ↑ 9.0 9.1 Medical Toxicology. Lippincott Williams & Wilkins. 2004. p. 684. ISBN 9780781728454. Archived from the original on 2017-07-07.
- ↑ 10.0 10.1 Carlsson, Bo, ed. (1997). Technological systems and industrial dynamics. Dordrecht: Kluwer Academic. p. 106. ISBN 9780792399728. Archived from the original on 2017-03-03.
- ↑ Fischer, Jnos; Ganellin, C. Robin (2006). Analogue-based Drug Discovery. John Wiley & Sons. p. 461. ISBN 9783527607495. Archived from the original on 8 September 2017. Retrieved 12 May 2020.
- ↑ World Health Organization (2019). World Health Organization model list of essential medicines: 21st list 2019. Geneva: World Health Organization. hdl:10665/325771. WHO/MVP/EMP/IAU/2019.06. License: CC BY-NC-SA 3.0 IGO.
- ↑ 13.0 13.1 "Single Drug Information – International Medical Products Price Guide". Archived from the original on 28 August 2021. Retrieved 12 February 2021.
- ↑ 14.0 14.1 "The Top 300 of 2020". ClinCalc. Archived from the original on 12 February 2021. Retrieved 11 April 2020.
- ↑ 15.0 15.1 "Metoprolol - Drug Usage Statistics". ClinCalc. 1 December 1981. Archived from the original on 11 April 2020. Retrieved 11 April 2020.
- ↑ Geffner DL, Hershman JM (July 1992). "Beta-adrenergic blockade for the treatment of hyperthyroidism". Am. J. Med. 93 (1): 61–8. doi:10.1016/0002-9343(92)90681-Z. PMID 1352658.
- ↑ "Metoprolol vs Toprol-XL Comparison". Drugs.com. 1 August 2019. Archived from the original on 25 September 2019. Retrieved 24 September 2019.
- ↑ "Metoprolol Tartrate vs. Metoprolol Succinate: A Comparison". Healthline. 28 February 2018. Archived from the original on 25 September 2019. Retrieved 24 September 2019.
- ↑ Eske, Jamie (25 September 2019). "Metoprolol tartrate vs. succinate: Differences in uses and effects". Medical News Today. Archived from the original on 25 September 2019. Retrieved 24 September 2019.
- ↑ 20.0 20.1 Morris J; Dunham A (2020). "Metoprolol". Statpearls. PMID 30422518.
- ↑ Costanzo L (2009). Physiology (3rd ed.). Saunders Elsevier. ISBN 978-1-4160-2320-3.
- ↑ Turner MD (April 2016). "Hyposalivation and Xerostomia: Etiology, Complications, and Medical Management". Dent. Clin. North Am. 60 (2): 435–43. doi:10.1016/j.cden.2015.11.003. PMID 27040294.
- ↑ "Metoprolol". Drugs.com. Archived from the original on 21 January 2010.
- ↑ Cruickshank JM (2010). "Beta-blockers and heart failure". Indian Heart Journal. 62 (2): 101–110. PMID 21180298.
- ↑ 25.0 25.1 25.2 "Metoprolol (Oral Route) Precautions". Drug Information. Mayo Clinic. Archived from the original on 2009-04-16.
- ↑ Page C, Hacket LP, Isbister GK (2009). "The use of high-dose insulin-glucose euglycemia in beta-blocker overdose: a case report". Journal of Medical Toxicology. 5 (3): 139–143. doi:10.1007/bf03161225. PMC 3550395. PMID 19655287.
- ↑ Albers S, Elshoff JP, Völker C, Richter A, Läer S (2005). "HPLC quantification of metoprolol with solid-phase extraction for the drug monitoring of pediatric patients". Biomedical Chromatography. 19 (3): 202–207. doi:10.1002/bmc.436. PMID 15484221.
- ↑ Baselt R (2008). Disposition of Toxic Drugs and Chemicals in Man (8th ed.). Foster City, CA: Biomedical Publications. pp. 1023–1025.
- ↑ 29.0 29.1 Suita, Kenji; Fujita, Takayuki; Hasegawa, Nozomi; Cai, Wenqian; Jin, Huiling; Hidaka, Yuko; Prajapati, Rajesh; Umemura, Masanari; Yokoyama, Utako (2015-07-23). "Norepinephrine-Induced Adrenergic Activation Strikingly Increased the Atrial Fibrillation Duration through β1- and α1-Adrenergic Receptor-Mediated Signaling in Mice". PLOS ONE. 10 (7): e0133664. Bibcode:2015PLoSO..1033664S. doi:10.1371/journal.pone.0133664. ISSN 1932-6203. PMC 4512675. PMID 26203906.
- ↑ Swaisland HC, Ranson M, Smith RP, Leadbetter J, Laight A, McKillop D, Wild MJ (2005). "Pharmacokinetic drug interactions of gefitinib with rifampicin, itraconazole and metoprolol". Clinical Pharmacokinetics. 44 (10): 1067–1081. doi:10.2165/00003088-200544100-00005. PMID 16176119.
- ↑ Blake CM, Kharasch ED, Schwab M, Nagele P (Sep 2013). "A meta-analysis of CYP2D6 metabolizer phenotype and metoprolol pharmacokinetics". Clin Pharmacol Ther. 94 (3): 394–9. doi:10.1038/clpt.2013.96. PMC 3818912. PMID 23665868.
- ↑ Cupp M (2009). "Alternatives for Metoprolol Succinate" (PDF). Pharmacist's Letter / Prescriber's Letter. 25 (250302). Archived (PDF) from the original on 20 September 2016. Retrieved 2012-07-06.
- ↑ Rote Liste Service GmbH (Hrsg.): Rote Liste 2017 – Arzneimittelverzeichnis für Deutschland (einschließlich EU-Zulassungen und bestimmter Medizinprodukte). Rote Liste Service GmbH, Frankfurt/Main, 2017, Aufl. 57, ISBN 978-3-946057-10-9, S. 200.
- ↑ The Medicines and Healthcare Products Regulatory Agency (2018). Medicines & Medical Devices Regulation. London, pp.1-24.
- ↑ Engelke LC, Ewell TB (2011). "The Ethics and Legality of Beta Blockers for Performance Anxiety: What Every Educator Should Know". College Music Symposium. 51. doi:10.18177/sym.2011.51.sr.18. ISSN 2330-2011. JSTOR 26513061.
- ↑ "PAR Metoprolol Tartrate 50 mg and 100 mg tablets" (PDF). Medicines and Healthcare products Regulatory Agency (MHRA). PL 30139/0017-18; UK/H/4414/001-2/DC. Archived (PDF) from the original on 11 May 2012. Retrieved 25 September 2019.
- ↑ "UKPAR Metoprolol Tartrate 50 mg and 100 mg Film-coated Tablets" (PDF). Medicines and Healthcare products Regulatory Agency (MHRA). PL 17907/0129-30. Archived (PDF) from the original on 25 September 2019. Retrieved 25 September 2019.
- ↑ Hughes D (October 2015). "The World Anti-Doping Code in sport: Update for 2015". Aust Prescr. 38 (5): 167–70. doi:10.18773/austprescr.2015.059. PMC 4657305. PMID 26648655.
- ↑ World Anti-Doping Agency (2017). Prohibited List. Montreal: WADA's Executive Committee.
- ↑ Barnes KP, Rainbow CR (September 2013). "Update on banned substances 2013". Sports Health. 5 (5): 442–7. doi:10.1177/1941738113478546. PMC 3752189. PMID 24427415.
- ↑ Politi L, Groppi A, Polettini A (2005). "Applications of liquid chromatography-mass spectrometry in doping control". J Anal Toxicol. 29 (1): 1–14. doi:10.1093/jat/29.1.1. PMID 15808007.
- ↑ "$11 Million Settlement Reached in Lawsuit Involving the Heart Medication, Toprol XL®, and its generic equivalent, metoprolol succinate". www.prnewswire.com. Archived from the original on 31 March 2018. Retrieved 30 March 2018.
External links
- "Metoprolol". Drug Information Portal. U.S. National Library of Medicine. Archived from the original on 6 February 2020. Retrieved 6 February 2020.
- Dean L (2017). "Metoprolol Therapy and CYP2D6 Genotype". In Pratt VM, McLeod HL, Rubinstein WS, et al. (eds.). Medical Genetics Summaries. National Center for Biotechnology Information (NCBI). PMID 28520381. Bookshelf ID: NBK425389. Archived from the original on 26 October 2020. Retrieved 6 February 2020.
| External sites: | |
|---|---|
| Identifiers: |
|
- Pages using duplicate arguments in template calls
- CS1 maint: unrecognized language
- Drugs with non-standard legal status
- Chemical articles with unknown parameter in Infobox drug
- Chemical articles without CAS registry number
- Articles without EBI source
- Chemical pages without ChemSpiderID
- Chemical pages without DrugBank identifier
- Articles without KEGG source
- Articles without UNII source
- Drugs missing an ATC code
- Drugboxes which contain changes to watched fields
- All articles with unsourced statements
- Articles with unsourced statements from January 2018
- Articles with invalid date parameter in template
- AstraZeneca brands
- Beta blockers
- Chemical substances for emergency medicine
- Isopropyl compounds
- N-isopropyl-phenoxypropanolamines
- Novartis brands
- World Health Organization essential medicines (alternatives)
- RTT

