Labetalol
 | |
| Names | |
|---|---|
| Pronunciation | /ləˈbɛtəlɔːl/ |
| Trade names | Normodyne, Trandate, others |
| |
| Clinical data | |
| Drug class | Beta blocker[1] |
| Main uses | High blood pressure[2] |
| Side effects | Low blood pressure with standing, dizziness, feeling tired, nausea[1] |
| Pregnancy category |
|
| Routes of use | By mouth, intravenous |
| Defined daily dose | 600 mg[3] |
| External links | |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a685034 |
| Legal | |
| License data | |
| Legal status |
|
| Pharmacokinetics | |
| Bioavailability | 25% |
| Protein binding | 50% |
| Metabolism | Liver pass metabolism, |
| Elimination half-life | Tablet: 6-8 hours; IV: 5.5 hours |
| Excretion | Excreted in urine, not removed by hemodialysis |
| Chemical and physical data | |
| Formula | C19H24N2O3 |
| Molar mass | 328.412 g·mol−1 |
| 3D model (JSmol) | |
| Chirality | Racemic mixture |
| |
| |
Labetalol is a medication used to treat high blood pressure and in long term management of angina.[1][4] This includes essential hypertension, hypertensive emergencies, and hypertension of pregnancy.[4] In essential hypertension it is generally less preferred than a number of other blood pressure medications.[1] It can be given by mouth or by injection into a vein.[1]
Common side effects include low blood pressure with standing, dizziness, feeling tired, and nausea.[1] Serious side effects may include low blood pressure, liver problems, heart failure, and bronchospasm.[1] Use appears safe in the latter part of pregnancy and it is not expected to cause problems during breastfeeding.[4][5] It works by blocking the activation of both α-receptors and β-receptors.[6]
Labetalol was patented in 1966 and came into medical use in 1977.[7] It is available as a generic medication.[4] A month supply of labetalol at 200mg three times daily, in the United Kingdom, costs the NHS less than £15 as of 2020.[4] In the United States the same dose for a month costs just less than US$24.[8] In 2017, it was the 211th most commonly prescribed medication in the United States, with more than two million prescriptions.[9][10]
Medical uses
Labetalol is effective in the management of hypertensive emergencies, postoperative hypertension, pheochromocytoma-associated hypertension, and rebound hypertension from beta blocker withdrawal. [11]
It has a particular indication in the treatment of pregnancy-induced hypertension,[6] which is commonly associated with pre-eclampsia. [12]
It is also used as an alternative in the treatment of severe hypertension.[11]
Children: no studies have established safety or usefulness.[13]
Elderly: the elderly are more likely to experience dizziness when taking labetalol. Labetalol should be dosed with caution in the elderly and counseled on this side effect.[13]
Dosage
For high blood pressure in pregnancy it is often started at a dose of 100 mg twice per day by mouth and is often increased to 400 to 800 mg per day with a maximum dose of 2,400 mg per day.[2]
By injection it is typically given at a dose of 20 mg over more than a minute and may be repeated after 10 minutes.[14] If this is insufficient than doses of 40 mg followed by 80 mg may be used to a maximum of 300 mg.[14]
The defined daily dose is 600 mg by mouth or by injection.[3]
Side effects
Common
- Neurologic: headache (2%), dizziness (11%) [13]
- Gastrointestinal: nausea (6%), dyspepsia (3%) [13]
- Cholinergic: nasal congestion (3%), ejaculation failure (2%) [13]
- Respiratory: dyspnea (2%) [13]
- Other: fatigue (5%), vertigo (2%), orthostatic hypotension [13]
Low blood pressure with standing is more severe and more common with IV formulation (58% vs 1%[13]) and is often the reason larger doses of the oral formulation cannot be used.[15]
Rare
- Fever [13]
- Muscle cramps [13]
- Dry eyes [13]
- Heart block [13]
- Hyperkalemia [13]
- Hepatotoxicity [13]
- Drug eruption similar to lichen planus[16]
- Hypersensitivity - which may result in a lethal respiratory distress[13]
Pregnancy and breastfeeding
Pregnancy: studies in lab animals showed no harm to the baby. However, a comparable well-controlled study has not been performed in pregnant women.[13]
Nursing: breast milk has been shown to contain small amounts of labetalol (0.004% original dose). Prescribers should be cautious in the use of labetalol for nursing mothers.[13]
Contraindications
Labetalol is contraindicated in people with overt cardiac failure, greater-than-first-degree heart block, severe bradycardia, cardiogenic shock, severe hypotension, anyone with a history of obstructive airway disease including asthma, and those with hypersensitivity to the drug.[17]
Pharmacology
Mechanism of action
Labetalol works by blocking the activation of both α-receptors and β-receptors.[6] At doses uses for treatment, it predominantly acts on β-receptors.[6]
In short-term, acute situations, labetalol reduces blood pressure by decreasing systemic vascular resistance with little effect on stroke volume, heart rate and cardiac output.[18] During long-term use, labetalol can reduce heart rate during exercise while maintaining cardiac output by an increase in stroke volume.[19] As well as being a dual alpha (α1) and beta (β1/β2) adrenergic receptor blocker, it competes with other Catecholamines for binding to these sites.[20] Its action on these receptors are potent and reversible.[17] Labetalol is highly selective for postsynaptic alpha1- adrenergic, and non-selective for beta-adrenergic receptors. It is about equipotent in blocking beta1- and beta2- receptors.[21]
The amount of alpha to beta blockade depends on whether labetalol is administered orally or intravenously (IV). Orally, the ratio of alpha to β blockade is 1:3. Intravenously, alpha to β blockade ratio is 1:7.[21][17] Thus, the labetalol can be thought to be a beta-blocker with some alpha-blocking effects.[17][20][22] By comparison, labetalol is a weaker β-blocker than propranolol, and has a weaker affinity for alpha-receptors compared to Phentolamine.[21][20]
Labetalol possesses intrinsic sympathomimetic activity.[22] In particular, it is a partial agonist at beta2- receptors located in the vascular smooth muscle. Labetalol relaxes vascular smooth muscle by a combination of this partial beta2- agonism and through alpha1- blockade.[22][23] Overall, this vasodilatory effect can decrease blood pressure.[24]
Similar to local anesthetics and sodium channel blocking antiarrhythmics, labetalol also has membrane stabilizing activity.[22][25] By decreasing sodium entry, labetalol decreases action potential firing and thus has local anesthetic activity.[26]
Physiological action
The physiological effects of labetalol when administered acutely (intravenously) are not predictable solely by their receptor blocking effect, i.e. blocking beta1- receptors should decrease heart rate, but labetalol does not. When labetalol is given in acute situations, it decreases the peripheral vascular resistance and systemic blood pressure while having little effect on the heart rate, cardiac output and stroke volume, despite its alpha1-, beta1- and beta2- blocking mechanism.[18][19] These effects are mainly seen when the person is in the upright position.[24]
Long term labetalol use also has different effects from other beta-blocking drugs. Other beta-blockers, such as propranolol, persistently reduce cardiac output during exercise. The peripheral vascular resistance decreases when labetalol is first administered. Continuous labetalol use further decreases peripheral vascular resistance. However, during exercise, cardiac output remains the same due to a compensatory mechanism that increases stroke volume. Thus, labetalol is able to reduce heart rate during exercise while maintaining cardiac output by the increase in stroke volume.[19]
Pharmacokinetics
Labetalol, in animal models, was found to cross the blood-brain-barrier in only negligible amounts.[27]
Chemistry
| Stereoisomers of labetalol | |
|---|---|
 (R,R)-Labetalol CAS number: 75659-07-3 |
 (S,S)-Labetalol CAS number: 83167-24-2 |
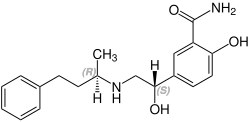 (R,S)-Labetalol CAS number: 83167-32-2 |
 (S,R)-Labetalol CAS number: 83167-31-1 |
The minimum requirement for adrenergic agents is a primary or secondary amine separated from a substituted benzene ring by one or two carbons.[28] This configuration results in strong agonist activity. As the size of the substituent attached to the amine becomes greater, particularly with respect to a t-butyl group, then the molecule typically is found to have receptor affinity without intrinsic activity, and is, therefore, an antagonist.[28] Labetalol, with its 1-methyl-3-phenylpropyl substituted amine, is greater in size relative to a t-butyl group and therefore acts predominantly as an antagonist. The overall structure of labetalol is very polar. This was created by substituting the isopropyl group in the standard beta-blocker structure with an aralkyl group, including a carboxamide group on the meta position, and by adding a hydroxyl group on the para position.[21]
Labetalol has two chiral carbons and consequently exists as four stereoisomers.[29] Two of these isomers, the (S,S)- and (R,S)- forms are inactive. The third, the (S,R)-isomer, is a powerful α1 blocker. The fourth isomer, the (R,R)-isomer which is also known as dilevalol, is a mixed nonselective β blocker and selective α1 blocker.[21] Labetalol is typically given as a racemic mixture to achieve both alpha and beta receptor blocking activity.[30]
Labetalol acts by blocking alpha and beta adrenergic receptors, resulting in decreased peripheral vascular resistance without significant alteration of heart rate or cardiac output.
The β:α antagonism of labetalol is approximately 3:1.[31][32]
It is chemically designated in International Union of Pure and Applied Chemistry (IUPAC) nomenclature as 2-hydroxy-5-[1-hydroxy-2-[(1-methyl-3-phenylpropyl)amino]ethyl]benzamide monohydrochloride.[30][33]
Society and culture
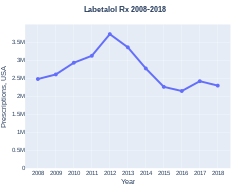
Cost
A month supply of labetalol at 200mg three times daily, in the United Kingdom, costs the NHS less than £15 as of 2020.[4] In the United States the same dose for a month costs just less than US$24.[8] In 2017, it was the 211th most commonly prescribed medication in the United States, with more than two million prescriptions.[9]
-
Labetalol costs (US)
-
Labetalol prescriptions (US)
History
Labetalol was the first drug created that combined both α-receptor and β-receptor adrenergic blocking properties. It was created to potentially fix the compensatory reflex issue that occurred when blocking a single receptor subtype, i.e. vasoconstriction after blocking beta-receptors or tachycardia after blocking alpha receptors. Because the reflex from blocking the single receptor subtypes acted to prevent the lowering of blood pressure, it was postulated that weak blocking of both alpha- and beta- receptors could work together to decrease blood pressure.[19][21]
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 1.6 "Labetalol Monograph for Professionals". Drugs.com. American Society of Health-System Pharmacists. Archived from the original on 15 February 2021. Retrieved 15 February 2021.
- ↑ 2.0 2.1 "LABETALOL oral - Essential drugs". medicalguidelines.msf.org. Archived from the original on 29 August 2021. Retrieved 31 August 2020.
- ↑ 3.0 3.1 "WHOCC - ATC/DDD Index". www.whocc.no. Archived from the original on 2 July 2020. Retrieved 31 August 2020.
- ↑ 4.0 4.1 4.2 4.3 4.4 4.5 BNF (80 ed.). BMJ Group and the Pharmaceutical Press. September 2020 – March 2021. p. 160-161. ISBN 978-0-85711-369-6.
{{cite book}}: CS1 maint: date format (link) - ↑ "Labetalol Use During Pregnancy". Drugs.com. Archived from the original on 29 November 2020. Retrieved 11 March 2019.
- ↑ 6.0 6.1 6.2 6.3 Ritter, Professor of Clinical Pharmacology Guy's King's and St Thomas's Medical Schools James M.; Flower, Rod J.; Henderson, Graeme; Loke, Yoon Kong; MacEwan, David; Rang, Humphrey P. (2020). "15. Noradrenergic transmission". Rang & Dale's Pharmacology. Elsevier. p. 210. ISBN 978-0-7020-7448-6. Archived from the original on 2021-08-28. Retrieved 2021-03-01.
- ↑ Fischer, Jnos; Ganellin, C. Robin (2006). Analogue-based Drug Discovery. John Wiley & Sons. p. 463. ISBN 9783527607495. Archived from the original on 2018-12-24. Retrieved 2019-03-01.
- ↑ 8.0 8.1 "Labetalol Prices, Coupons & Patient Assistance Programs". Drugs.com. Archived from the original on 15 February 2021. Retrieved 15 February 2021.
- ↑ 9.0 9.1 "The Top 300 of 2020". ClinCalc. Archived from the original on 12 February 2021. Retrieved 11 April 2020.
- ↑ "Labetalol - Drug Usage Statistics". ClinCalc. Archived from the original on 11 April 2020. Retrieved 11 April 2020.
- ↑ 11.0 11.1 Koda-Kimble, Mary A.; Alldredge, Brian K. (2013). "21". Koda-Kimble and Young's Applied Therapeutic: The Clinical Use of Drugs. Philadelphia: Philadelphia: Lippincott Williams & Wilkins. ISBN 978-1-60913-713-7.
- ↑ Arulkumaran, N; Lightstone, L (December 2013). "Severe pre-eclampsia and hypertensive crises". Best Practice & Research. Clinical Obstetrics & Gynaecology. 27 (6): 877–84. doi:10.1016/j.bpobgyn.2013.07.003. PMID 23962474.
- ↑ 13.00 13.01 13.02 13.03 13.04 13.05 13.06 13.07 13.08 13.09 13.10 13.11 13.12 13.13 13.14 13.15 13.16 "Trandate" (PDF). Prometheus Laboratories Inc. November 2010. Archived (PDF) from the original on 7 August 2016. Retrieved 3 November 2015.
- ↑ 14.0 14.1 "LABETALOL injectable - Essential drugs". medicalguidelines.msf.org. Archived from the original on 29 August 2021. Retrieved 2 September 2020.
- ↑ "Labetalol hydrochloride" (PDF). Hospira. May 2015. Archived (PDF) from the original on 4 March 2016. Retrieved 3 November 2015.
- ↑ Shiohara T, Kano Y (2007). "Lichen planus and lichenoid dermatoses". In Bolognia JL (ed.). Dermatology. St. Louis: Mosby. p. 161. ISBN 978-1-4160-2999-1.
- ↑ 17.0 17.1 17.2 17.3 "Labetalol [package insert]. Spring Valley, NY: Par Pharmaceutical; 2011" (PDF). Archived from the original (PDF) on 2015-12-10. Retrieved 2015-11-03.
- ↑ 18.0 18.1 MacCarthy, E. P.; Bloomfield, S. S. (1983-08-01). "Labetalol: a review of its pharmacology, pharmacokinetics, clinical uses and adverse effects". Pharmacotherapy. 3 (4): 193–219. doi:10.1002/j.1875-9114.1983.tb03252.x. ISSN 0277-0008. PMID 6310529.
- ↑ 19.0 19.1 19.2 19.3 Louis, W. J.; McNeil, J. J.; Drummer, O. H. (1984-01-01). "Pharmacology of combined alpha-beta-blockade. I". Drugs. 28 Suppl 2: 16–34. doi:10.2165/00003495-198400282-00003. ISSN 0012-6667. PMID 6151889.
- ↑ 20.0 20.1 20.2 Robertson, D; Biaggioni, I (2012). Katzung, BG (ed.). Adrenoceptor Antagonist Drugs IN: Basic & Clinical Pharmacology (12 ed.). San Francisco: McGraw Hill Lange Medical. pp. 151–168. ISBN 978-0-07-176401-8.
- ↑ 21.0 21.1 21.2 21.3 21.4 21.5 Louis, W.J.; McNeill, JJ; Drummer, OH (1988). Doyle, AE (ed.). Labetalol and other vasodilator/Beta-blocking drugs. IN: Handbook of Hypertension. Amsterdam, Netherlands: Elsevier Sciences Publishing Co. pp. 246–273. ISBN 978-0-444-90469-0.
- ↑ 22.0 22.1 22.2 22.3 Westfall, David P (2004). Craig, Charles R (ed.). Adrenoreceptor Antagonists IN: Modern Pharmacology with Clinical Applications (6th ed.). Baltimore, MD: Lippincott Williams & Wilkins. pp. 109–117. ISBN 978-0781737623.
- ↑ Lund-Johansen, P. (1988-01-01). "Hemodynamic effects of beta-blocking compounds possessing vasodilating activity: a review of labetalol, prizidilol, and dilevalol". Journal of Cardiovascular Pharmacology. 11 Suppl 2: S12–17. doi:10.1097/00005344-198800000-00004. ISSN 0160-2446. PMID 2464093.
- ↑ 24.0 24.1 Lund-Johansen, P. (1984-01-01). "Pharmacology of combined alpha-beta-blockade. II. Haemodynamic effects of labetalol". Drugs. 28 Suppl 2: 35–50. doi:10.2165/00003495-198400282-00004. ISSN 0012-6667. PMID 6151890.
- ↑ Mottram, Allan R.; Erickson, Timothy B. (2009). Field, John (ed.). Toxicology in Emergency Cardiovascular Care IN: The Textbook of Emergency Cardiovascular Care and CPR. Philadelphia, PA: Lippincott WIlliams & Wilkins. pp. 443–452. ISBN 978-0-7817-8899-1.
- ↑ Exam Zone (1 January 2009). Elsevier Comprehensive Guide. Elsevier India. pp. 449–. ISBN 978-81-312-1620-0.
- ↑ Detlev Ganten; Patrick J. Mulrow (6 December 2012). Pharmacology of Antihypertensive Therapeutics. Springer Science & Business Media. pp. 147–. ISBN 978-3-642-74209-5.
- ↑ 28.0 28.1 "Medicinal Chemistry of Adrenergics and Cholinergics". Archived from the original on 2010-11-04. Retrieved 2010-10-16.
- ↑ Riva E, Mennini T, Latini R (December 1991). "The alpha- and beta-adrenoceptor blocking activities of labetalol and its RR-SR (50:50) stereoisomers". Br. J. Pharmacol. 104 (4): 823–8. doi:10.1111/j.1476-5381.1991.tb12513.x. PMC 1908821. PMID 1687367.
- ↑ 30.0 30.1 Robertson D, Biaggioni, I. Adrenoceptor Antagonist Drugs. In: Katzung BG, Masters SB, Trevor AJ, eds. Basic & Clinical Pharmacology. 12th ed. San Francisco, CA: McGraw Hill Lange Medical; 2012: 151-168. ISBN 978-0-07-176401-8.
- ↑ Katzung, Bertram G. (2006). Basic and clinical pharmacology. New York: McGraw-Hill Medical. p. 170. ISBN 978-0-07-145153-6.
- ↑ D A Richards; J Tuckman; B N Prichard (October 1976). "Assessment of alpha- and beta-adrenoceptor blocking actions of labetalol". Br J Clin Pharmacol. 3 (5): 849–855. doi:10.1111/j.1365-2125.1976.tb00637.x. PMC 1428931. PMID 9968.
- ↑ "labetalol | C19H24N2O3 - PubChem". pubchem.ncbi.nlm.nih.gov. Archived from the original on 2016-03-23. Retrieved 2015-11-04.
External links
| Identifiers: |
|
|---|
- "Labetalol". Drug Information Portal. U.S. National Library of Medicine. Archived from the original on 2020-10-30. Retrieved 2019-03-11.
- Pages using duplicate arguments in template calls
- CS1 maint: date format
- CS1: long volume value
- Drugs with non-standard legal status
- Chemical articles with unknown parameter in Infobox drug
- Chemical articles without CAS registry number
- Articles without EBI source
- Chemical pages without ChemSpiderID
- Chemical pages without DrugBank identifier
- Articles without KEGG source
- Articles without UNII source
- Drugs missing an ATC code
- Drugboxes which contain changes to watched fields
- Beta blockers
- Salicylamides
- Phenylethanolamines
- Hepatotoxins
- Peripherally selective drugs
- RTT