Serotonin–norepinephrine reuptake inhibitor
| Serotonin–norepinephrine reuptake inhibitor | |
|---|---|
| Drug class | |
 Chemical structure of duloxetine, an SNRI. | |
| Names | |
| Stem | -axine, -oxetine[1][2] |
| Other names | Serotonin–noradrenaline reuptake inhibitor (SNaRI), selective serotonin-norepinephrine reuptake Inhibitors (SSNRIs) |
| Clinical data | |
| Uses | Major depressive, generalized anxiety disorder, fibromyalgia[3][4] |
| Side effects | Constipation, sleepiness, dry mouth, sexual dysfunction, suicide, low sodium, bleeding, high blood pressure, serotonin syndrome[5][3] |
| Interactions | MOA inhibitors[6] |
| Common types | Venlafaxine, desvenlafaxime, duloxetine, milnacipran, levomilnacipran[5] |
| Biological target | Serotonin transporter; norepinephrine transporter[6] |
| External links | |
| Drugs.com | Drug Classes |
Serotonin–norepinephrine reuptake inhibitors (SNRIs) are a class of medication primarily used for major depressive and generalized anxiety disorder, when SSRIs are not effective.[4][7] Other uses may include fibromyalgia.[3] There is no clear evidence they are more effective than SSRIs; however, there are concerns of greater side effects.[3]
Common side effects include constipation, sleepiness, dry mouth, and sexual dysfunction.[5] Other side effects may include suicide, particularly in those under the age of 25, low sodium, bleeding, high blood pressure, and serotonin syndrome.[5][3] Stopping suddenly can result in withdrawal.[5] They are believed to work by blocking the reuptake of serotonin and norepinephrine by neurons.[5]
The first SNRI, venlafaxine, was approved for medical use in the United States in 1993.[5][8] Some are available as generic medication and are relatively inexpensive.[4] In 2018 duloxetine was the 36th and velafaxine was the 50th most commonly prescribed medication in the United States.[9]
Types

There are eight FDA approved SNRIs in the United States, with venlafaxine being the first to be developed in 1993. They vary by their other uses, chemical structure, side effects, and efficacy.[10]
Approval years for SNRIs (in the US):[10]
- 1993: Venlafaxine
- 1998: Sibutramine
- 2004: Duloxetine
- 2008: Desvenlafaxine
- 2009: Milnacipran
- 2013: Levomilnacipran
| Medication | Brand name | FDA Indications | Approval Year | Chemical structure | Notes |
|---|---|---|---|---|---|
| Atomoxetine | Strattera | 2002 | 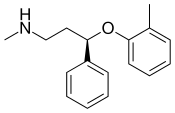 |
A norepinephrine-predominant SNRI used in the treatment of ADHD and, off-label, major depression. Was approved by FDA in 2002. Originally considered to be a selective norepinephrine reuptake inhibitor, but research subsequently revealed that it significantly inhibits the reuptake of serotonin at clinical dosages as well.[12] | |
| Desvenlafaxine[13] | Pristiq
Khedezla (ER) |
|
2007 |  |
The active metabolite of venlafaxine. It is believed to work in a similar manner, though some evidence suggests lower response rates compared to venlafaxine and duloxetine. It was introduced by Wyeth in May 2008 and was then the third approved SNRI.[14] |
| Duloxetine[15] | Cymbalta
Irenka |
|
2004 |  |
Approved for the treatment of depression and neuropathic pain in August 2004. Duloxetine is contraindicated in patients with heavy alcohol use or chronic liver disease, as duloxetine can increase the levels of certain liver enzymes that can lead to acute hepatitis or other diseases in certain at risk patients. Currently, the risk of liver damage appears to be only for patients already at risk, unlike the antidepressant nefazodone, which, though rare, can spontaneously cause liver failure in healthy patients.[19] Duloxetine is also approved for major depressive disorder (MDD), generalized anxiety disorder (GAD), diabetic neuropathy, chronic musculoskeletal pain, including chronic osteoarthritis pain and chronic low back pain.[17] Duloxetine also undergoes hepatic metabolism and has been shown to cause inhibition of the hepatic cytochrome P450 enzyme CYP 2D6.[20] Caution should be taking Duloxetine with other medications that are metabolized by CYP 2D6 as this may precipitate a potential drug-drug interaction.[20] |
| Levomilnacipran | Fetzima |
|
2013 | 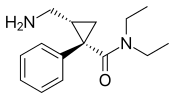 |
The levorotating isomer of milnacipran. Under development for the treatment of depression in the United States and Canada, it was approved by the FDA for treatment of MDD in July 2013. |
| Milnacipran | Ixel Savella Impulsor |
|
1996 |  |
Shown to be significantly effective in the treatment of depression and fibromyalgia.[21] The Food and Drug Administration (FDA) approved milnacipran for treatment of fibromyalgia in the United States of America in January 2009, however it is currently not approved for depression in that country. Milnacipran has been commercially available in Europe and Asia for several years. It was first introduced in France in 1996. |
| Sibutramine | Meridia | 1997 |  |
An SNRI, which, instead of being developed for the treatment of depression, was widely marketed as an appetite suppressant for weight loss purposes. Sibutramine was the first drug for the treatment of obesity to be approved in 30 years.[23] It has been associated with increased cardiovascular events and strokes and has been withdrawn from the market in several countries and regions including the United States in 2010.[24] | |
| Tramadol | Ultram |
|
1977 |  |
A dual weak opioid and SNRI. It was approved by the FDA in 1995, though it has been marketed in Germany since 1977. The drug is used to treat acute and chronic pain. It has shown effectiveness in the treatment of fibromyalgia, though it is not specifically approved for this purpose. The drug is also under investigation as an antidepressant and for the treatment of neuropathic pain. It is related in chemical structure to venlafaxine. Due to being an opioid, there is risk of abuse and addiction, but it does have less abuse potential, respiratory depression, and constipation compared to other opioids (hydrocodone, oxycodone, etc.).[25] |
| Venlafaxine | Effexor | 1994 |  |
The first and most commonly used SNRI. It was introduced by Wyeth in 1994. The reuptake effects of venlafaxine are dose-dependent. At low doses (<150 mg/day), it acts only on serotonergic transmission. At moderate doses (>150 mg/day), it acts on serotonergic and noradrenergic systems, whereas at high doses (>300 mg/day), it also affects dopaminergic neurotransmission.[28] At small doses, venlafaxine has also been shown to be effective in treating vasomotor symptoms (hot flashes and night sweats) of menopause and may be as effective as hormone replacement therapy (HRT).[27] |
Medical uses
SNRIs have been tested for treatment of the following conditions:
- Major depressive disorder (MDD)
- Posttraumatic stress disorder (PTSD)
- Generalized anxiety disorder (GAD)
- Social anxiety disorder (SAD)
- Obsessive compulsive disorder[29][30][31]
- Panic disorder
- Neuropathic pain
- Fibromyalgia
- Chronic musculoskeletal pain
In some studies, SNRIs demonstrated slightly higher antidepressant efficacy than the SSRIs (response rates 63.6% versus 59.3%).[32] However, in one study escitalopram had a superior efficacy profile to venlafaxine.[33]
Pregnancy
Currently, no antidepressants are FDA approved during pregnancy. All SSRIs and SNRIs are Category C, except paroxetine, which is Category D since it has shown association with congenital heart disorders.[34] Use of antidepressants during pregnancy may result in fetus abnormalities affecting functional development of the brain and behavior.[34] Untreated depression may also affect birth outcomes, so it is recommended to discuss options with a provider to weigh the risks and benefits.
Pediatrics
SSRIs and SNRIs have been shown to be effective in treating major depressive disorder and anxiety in pediatric populations.[35] However, there is a risk of increased suicidality in pediatric populations for treatment of major depressive disorder, especially with venlafaxine.[35] Fluoxetine is the only antidepressant that is approved for child/adolescent major depressive disorder.[36]
Geriatrics
Most antidepressants, including SNRIs, are safe and effective in the geriatric population. Decisions are often based on co-morbid conditions, drug interactions, and patient tolerance. Due to differences in body composition and metabolism, starting doses are often half that of the recommended dose for younger adults.[37]
Contraindications
SNRIs are contraindicated in patients taking MAOIs within the last two weeks due to the increased risk of serotonin syndrome, which can be life-threatening.[38] Other drugs and substances that should be avoided due to increased risk of serotonin syndrome when combined with an SNRI include: other anti-depressants, anti-convulsants, analgesics, antiemetic agents, anti-migraine medications, methylene blue, linezolid, Lithium, St. John's worts, ecstasy, and LSD.[38] Signs and symptoms of serotonin syndrome include: hyperthermia, rigidity, myoclonus, autonomic instability with fluctuating vital signs, and mental status changes that include extreme agitation progressing to delirium and coma.[17]
Due to the effects of increased norepinephrine levels and, therefore, higher noradrenergic activity, pre-existing hypertension should be controlled before treatment with SNRIs and blood pressure periodically monitored throughout treatment.[39] Duloxetine has also been associated with cases of liver failure and should not be prescribed to patients with chronic alcohol use or liver disease. Studies have found that Duloxetine can increase liver function tests three times above their upper normal limit.[40] Patients suffering from coronary artery disease should caution the use of SNRIs.[41] Furthermore, due to some SNRIs' actions on obesity, patients with major eating disorders such as anorexia nervosa or bulimia should not be prescribed SNRIs.[22] Duloxetine and milnacipran are also contraindicated in patients with uncontrolled narrow-angle glaucoma, as they have been shown to increase incidence of mydriasis.[17][42]
Side effects
Because the SNRIs and SSRIs act in similar ways to elevate serotonin levels, they share many side effects, though to varying degrees. The most common side effects include nausea/vomiting, sweating, loss of appetite, dizziness, headache, increase in suicidal thoughts, and sexual dysfunction.[43] Elevation of norepinephrine levels can sometimes cause anxiety, mildly elevated pulse, and elevated blood pressure. However, norepinephrine-selective antidepressants, such as reboxetine and desipramine, have successfully treated anxiety disorders.[44] People at risk for hypertension and heart disease should monitor their blood pressure.[22][45][46][17][42] The side effects of upset stomach may be decreased by taking SNRIs with food.
Sexual Dysfunction
SNRIs, similarly to SSRIs, can cause several types of sexual dysfunction, such as erectile dysfunction, decreased libido, sexual anhedonia, and anorgasmia.[47][48][49] The two common sexual side effects are diminished interest in sex (libido) and difficulty reaching climax (anorgasmia), which are usually somewhat milder with SNRIs compared to SSRIs.[50] To manage sexual dysfunction, studies have shown that switching to or augmenting with bupropion or adding a PDE5 Inhibitor have decreased symptoms of sexual dysfunction.[51] Studies have shown that PDE5 Inhibitors, such as sildenafil (Viagra), tadalafil (Cialis), vardenafil (Levitra), and avanafil (Stendra), have sometimes been helpful to decrease the sexual dysfunction, including erectile dysfunction, although they have been shown to be more effective in men than women.[51]
Serotonin Syndrome
A serious, but rare, side effect of SNRIs is serotonin syndrome, which is caused by an excess of serotonin in the body. Serotonin syndrome can be caused by taking multiple serotonergic drugs, such as SSRIs or SNRIs. Other drugs that contribute to serotonin syndrome include MAO inhibitors, linezolid, tedizolid, methylene blue, procarbazine, amphetamines, clomipramine, and more.[52] Early symptoms of serotonin syndrome may include nausea, vomiting, diarrhea, sweating, agitation, confusion, muscle rigidity, dilated pupils, hyperthermia, rigidity, and goose bumps. More severe symptoms include fever, seizures, irregular heartbeat, delirium, and coma.[53][54][17] If signs or symptoms arise, discontinue treatment with serotonergic agents immediately.[53] It is recommended to washout 4 to 5 half-lives of the serotonergic agent before using an MAO inhibitor.[55]
Bleeding
Some studies suggest there are risks of upper gastrointestinal bleeding, especially venlafaxine, due to impairment of platelet aggregation and depletion of platelet serotonin levels.[56][57] Similarly to SSRIs, SNRIs may interact with anticoagulants, like warfarin. Currently, there is more evidence of SSRIs having higher risk of bleeding than SNRIs.[56] Studies have suggested caution when using SNRIs or SSRIs with high doses of nonsteroidal anti-inflammatory drugs (NSAIDs), such as ibuprofen or naproxen due to an increased risk of upper GI bleeding.[58]
Starting an SNRI regimen
Due to the changes in noradrenergic activity produced from norepinephrine and serotonin reuptake inhibition, patients that are just starting an SNRI regimen are usually given lower doses than their expected final dosing to allow the body to acclimate to the drug's effects. As the patient continues along at low doses without any side-effects, the dose is incrementally increased until the patient sees improvement in symptoms without detrimental side-effects.[59]
Discontinuation syndrome
As with SSRIs, the abrupt discontinuation of an SNRI usually leads to withdrawal, or "discontinuation syndrome", which could include states of anxiety and other symptoms. Therefore, it is recommended that users seeking to discontinue an SNRI slowly taper the dose under the supervision of a professional. Discontinuation syndrome has been reported to be markedly worse for venlafaxine when compared to other SNRIs. As such, as tramadol is related to venlafaxine, the same conditions apply.[60] This is likely due to venlafaxine's relatively short half-life and therefore rapid clearance upon discontinuation. In some cases, switching from venlafaxine to fluoxetine, a long-acting SSRI, and then tapering off fluoxetine, may be recommended to reduce discontinuation symptoms.[61][62] Signs and symptoms of withdrawal from abrupt cessation of an SNRI include dizziness, anxiety, insomnia, nausea, sweating, and flu-like symptoms, such as lethargy and malaise.[63]
Overdose
Causes
Overdosing on SNRIs can be caused by either drug combinations or excessive amounts of the drug itself. Venlafaxine is marginally more toxic in overdose than duloxetine or the SSRIs.[22][45][46][17][42][64] Risk of overdose is increased in patients taking multiple serotonergic agents or interacting agents.
Symptoms
Symptoms of SNRI overdose, whether it be a mixed drug interaction or the drug alone, vary in intensity and incidence based on the amount of medicine taken and the individuals sensitivity to SNRI treatment. Symptoms may include:[17]
- Somnolence
- Coma
- Serotonin syndrome
- Seizures
- Syncope
- Tachycardia
- Hypotension
- Hypertension
- Hyperthermia
- Vomiting
Management
Overdose is usually treated symptomatically, especially in the case of serotonin syndrome, which requires treatment with cyproheptadine and temperature control based on the progression of the serotonin toxicity.[65] Patients are often monitored for vitals and airways cleared to ensure that they are receiving adequate levels of oxygen. Another option is to use activated carbon in the GI tract in order to absorb excess neurotransmitter.[17] It is important to consider drug interactions when dealing with overdose patients, as separate symptoms can arise.
Pharmacology
Route of administration
SNRIs are delivered orally, usually in the form of capsules or tablets. It is recommended to take SNRIs in the morning with breakfast, which does not affect drug levels, but may help with certain side effects.[66] Norepinephrine has activating effects in the body and therefore can cause insomnia in some patients if taken at bedtime.[67] SNRIs can also cause nausea, which is usually mild and goes away within a few weeks of treatment, but taking the medication with food can help alleviate this.[68] The drugs themselves are usually a fine crystalline powder that diffuses into the body during digestion.
Mechanism of action
Monoamines are connected to the pathophysiology of depression. Symptoms may occur because concentrations of neurotransmitters, such as norepinephrine and serotonin, are insufficient, leading to downstream changes.[16][69] Medications for depression affect the transmission of serotonin, norepinephrine, and dopamine.[16] Older and more unselective antidepressants like TCAs and MAOIs inhibit the reuptake or metabolism of norepinephrine and serotonin in the brain, which results in higher concentrations of neurotransmitters.[69] Antidepressants that have dual mechanisms of action inhibit the reuptake of both serotonin and norepinephrine and, in some cases, inhibit with weak effect the reuptake of dopamine.[16] Antidepressants affect variable neuronal receptors like muscarinic-cholinergic, α1- and α2-adrenergic, and H1-histaminergic receptors, and sodium channels in the cardiac muscle, leading to decreased cardiac conduction and cardiotoxicity {source needed}. Selectivity of antidepressant agents are based on the neurotransmitters that are thought to influence symptoms of depression.[70] Drugs that selectively block the reuptake of serotonin and norepinephrine effectively treat depression and are better tolerated than TCAs. TCAs have comprehensive effects on various neurotransmitters receptors, which leads to lack of tolerability and increased risk of toxicity.[71]
Agents with dual serotonin and norepinephrine reuptake inhibition (SNRIs) are sometimes called non-tricyclic serotonin and norepinephrine reuptake inhibitors. Clinical studies suggest that compounds that increase the concentration in the synaptic cleft of both norepinephrine and serotonin are more successful than single acting agents in the treatment of depression, but the data is not conclusive whether SNRIs are a more effective treatment option over SSRIs for depression.[72][73][58] Dual reuptake inhibitors have low affinity at neuronal receptors of the other neurotransmitters, which have low adverse effects compared with the TCAs. Nontricyclic antidepressants have improved potency and onset action acceleration in antidepressant response than SSRIs alone, which give the impression that synergism is an efficient property in mediating antidepressant activity.
The non-tricyclic SNRIs have several important differences that are based on pharmacokinetics, metabolism to active metabolites, inhibition of CYP isoforms, effect of drug-drug interactions, and the half-life of the nontricyclic SNRIs.[74][75]
Combination of mechanisms of action in a single active agent is an important development in psychopharmacology.[75]
Mode of action
The condition for which SNRIs are mostly indicated, major depressive disorder, is thought to be mainly caused by decreased levels of serotonin and norepinephrine in the synaptic cleft, causing erratic signaling. Based on the monoamine hypothesis of depression, which asserts that decreased concentrations of monoamine neurotransmitters leads to depressive symptoms, the following relations were determined: "Norepinephrine may be related to alertness and energy as well as anxiety, attention, and interest in life; [lack of] serotonin to anxiety, obsessions, and compulsions; and dopamine to attention, motivation, pleasure, and reward, as well as interest in life."[76] SNRIs work by inhibiting the reuptake of the neurotransmitters serotonin and norepinephrine. This results in increased extracellular concentrations of serotonin and norepinephrine and, consequently, an increase in neurotransmission. Most SNRIs including venlafaxine, desvenlafaxine, and duloxetine, are several fold more selective for serotonin over norepinephrine, while milnacipran is three times more selective for norepinephrine than serotonin. Elevation of norepinephrine levels is thought to be necessary for an antidepressant to be effective against neuropathic pain, a property shared with the older tricyclic antidepressants (TCAs), but not with the SSRIs.[77]
Recent studies have shown that depression may be linked to increased inflammatory response,[78] thus attempts at finding an additional mechanism for SNRIs have been made. Studies have shown that SNRIs as well as SSRIs have significant anti-inflammatory action on microglia[79] in addition to their effect on serotonin and norepinephrine levels. As such, it is possible that an additional mechanism of these drugs that acts in combination with the previously understood mechanism exist. The implication behind these findings suggests use of SNRIs as potential anti-inflammatories following brain injury or any other disease where swelling of the brain is an issue. However, regardless of the mechanism, the efficacy of these drugs in treating the diseases for which they have been indicated has been proven, both clinically and in practice.[improper synthesis?]
Pharmacodynamics
Most SNRIs function alongside primary metabolites and secondary metabolites in order to inhibit reuptake of serotonin, norepinepherine, and marginal amounts of dopamine. For example, venlafaxine works alongside its primary metabolite O-desmethylvenlafaxine to strongly inhibit serotonin and norepinephrine reuptake in the brain. The evidence also suggests that dopamine and norepinepherine behave in a co-transportational manner, due to the inactivation of dopamine by norepinephrine reuptake in the frontal cortex, an area of the brain largely lacking in dopamine transporters. This effect of SNRIs results in increased dopamine neurotransmission, in addition to the increases in serotonin and norepinephrine activity.[80] Furthermore, because SNRIs are extremely selective, they have no measurable effects on other, unintended receptors, in contrast to monoamine oxidase inhibition.[81] Pharmaceutical tests have determined that use of both SNRIs or SSRIs can generate significant anti-inflammatory action on microglia, as well.[79][22][45][46][17][42]
Activity profiles
| Compound | SERT | NET | ~Ratio (5-HT : NE) | ||
|---|---|---|---|---|---|
| Ki | IC50 | Ki | IC50 | ||
| Venlafaxine | 7.8 | 145 | 1,920 | 1420 | 30:1 |
| Des-venlafaxine | 40.2 | 47.3 | 558.4 | 531.3 | 14:1 |
| Duloxetine | 0.07 | 3.7 | 1.17 | 20 | 10:1 |
| Atomoxetine | 87[83] | 5.4 [83] | 0.06:1 (1:16) | ||
| Milnacipran | 8.44 | 151 | 22 | 68 | 1.6:1 |
| Levo-milnacipran | 11.2 | 19.0 | 92.2 | 10.5 | 1:2 |
| All of the Ki and IC50 values are nM. The 5-HT/NE ratio is based on IC50 values for the SERT and NET.[82] | |||||
Structure activity relationship
Aryloxypropanamine scaffold
Several reuptake inhibitors contain an aryloxypropanamine scaffold. This structural motif has potential for high affinity binding to biogenic amine transports.[75] Drugs containing an aryloxypropanamine scaffold have selectivity profile for norepinephrine and serotonin transporters that depends on the substitution pattern of the aryloxy ring. Selective NRIs contain a substituent in 2' position of the aryloxy ring but SSRIs contain a substituent in 4' position of the aryloxy ring. Atomoxetine, nisoxetine and reboxetine all have a substitution group in the 2' position and are selective NRIs while compounds that have a substitution group in the 4' position (like fluoxetine and paroxetine) are SSRIs. Duloxetine contains a phenyl group fused at the 2' and 3' positions, therefore it has dual selective norepinephrine and serotonin reuptake inhibitory effects and has similar potencies for the both transporters.[85] The nature of the aromatic substituent also has a significant influence on the activity and selectivity of the compounds as inhibitors of the serotonin or the norepinephrine transporters.[75]

Cycloalkanol ethylamine scaffold
Venlafaxine and desvenlafaxine contain a cycloalkanol ethylamine scaffold. Increasing the electron-withdrawing nature of the aromatic ring provides a more potent inhibitory effect of norepinephrine uptake and improves the selectivity for norepinephrine over the serotonin transporter.[85] Effects of chloro, methoxy and trifluoromethyl substituents in the aromatic ring of cycloalkanol ethylamine scaffold were tested. The results showed that the strongest electron-withdrawing m-trifluoromethyl analogue exhibited the most potent inhibitory effect of norepinephrine and the most selectivity over serotonin uptake.[85] WY-46824, a piperazine-containing derivative, has shown norepinephrine and dopamine reuptake inhibition. Further synthesis and testing identified WAY-256805, a potent norepinephrine reuptake inhibitor that exhibited excellent selectivity and was efficacious in animal models of depression, pain, and thermoregulatory dysfunction.[86]

Milnacipran

Milnacipran is structurally different from other SNRIs.[74] The SAR of milnacipran derivatives at transporter level is still largely unclear and is based on in vivo efficacy that was reported in 1987. N-methylation of milnacipran in substituent group R4 and R5 reduces the norepinephrine and serotonin activity.[87] Researches on different secondary amides in substitution groups R6 and R7 showed that π electrons play an important role in the interaction between transporters and ligands. A phenyl group in substituent R6 showed effect on norepinephrine transporters. Substituent groups in R6 and R7 with allylic double bond showed significant improved effect on both norepinephrine and serotonin transporters.[87] Studies show that introducing a 2-methyl group in substituent R3, the potency at norepinephrine and serotonin transporters are almost abolished. Methyl groups in substituent groups R1 and R2 also abolish the potency at norepinephrine and serotonin transporters. Researchers found that replacing one of the ethyl groups of milnacipran with an allyl moiety increases the norepinephrine potency.[88] The pharmacophore of milnacipran derivatives is still largely unclear.[87]
The conformation of milnacipran is an important part of its pharmacophore. Changing the SAR in milnacipran changes the stereochemistry of the compound and affects the norepinephrine and serotonin concentration. Milnacipran is marketed as a racemic mixture. Effects of milnacipran reside in the (1S,2R)-isomer and substitution of the phenyl group in the (1S,2R)-isomer has negative impact on norepinephrine concentration.[88] Milnacipran has low molecular weight and low lipophilicity. Because of these properties, milnacipran exhibits almost ideal pharmacokinetics in humans such as high bioavailability, low inter-subject variability, limited liver enzyme interaction, moderate tissue distribution and a reasonably long elimination half-life. Milnacipran's lack of drug-drug interactions via cytochrome P450 enzymes is thought to be an attractive feature because many of the central nervous system drugs are highly lipophilic and are mainly eliminated by liver enzymes.[88]
Pharmacokinetics
The half-life of venlafaxine is about 5 hours, and with once-daily dosing, steady-state concentration is achieved after about 3 days, though its active metabolite desvenlafaxine lasts longer.[46] The half-life of desvenlafaxine is about 11 hours, and steady-state concentrations are achieved after 4 to 5 days.[45] The half-life of duloxetine is about 12 hours (range: 8–17 hours), and steady-state is achieved after about 3 days.[17] Milnacipran has a half-life of about 6 to 8 hours, and steady-state levels are reached within 36 to 48 hours.[42]
History
In 1952, iproniazid, an antimycobacterial agent, was discovered to have psychoactive properties while researched as a possible treatment for tuberculosis. Researchers noted that patients given iproniazid became cheerful, more optimistic, and more physically active. Soon after its development, iproniazid and related substances were shown to slow enzymatic breakdown of serotonin, dopamine, and norepinephrine via inhibition of the enzyme monoamine oxidase. For this reason, this class of drugs became known as monoamine oxidase inhibitors, or MAOIs. During this time development of distinctively different antidepressant agents was also researched. Imipramine became the first clinically useful tricyclic antidepressant (TCA). Imipramine was found to affect numerous neurotransmitter systems and to block the reuptake of norepinephrine and serotonin from the synapse, therefore increasing the levels of these neurotransmitters. Use of MAOIs and TCAs gave major advances in treatment of depression but their use was limited by unpleasant side effects and significant safety and toxicity issues.[89]
Throughout the 1960s and 1970s, the catecholamine hypothesis of emotion and its relation to depression was of wide interest and that the decreased levels of certain neurotransmitters, such as norepinephrine, serotonin, and dopamine might play a role in the pathogenesis of depression. This led to the development of fluoxetine, the first SSRI. The improved safety and tolerability profile of the SSRIs in patients with MDD, compared with TCAs and MAOIs, represented yet another important advance in the treatment of depression.[89]
Since the late 1980s, SSRIs have dominated the antidepressant drug market. Today, there is increased interest in antidepressant drugs with broader mechanisms of action that may offer improvements in efficacy and tolerability. In 1993, a new drug was introduced to the US market called venlafaxine, a serotonin–norepinephrine reuptake inhibitor.[26] Venlafaxine was the first compound described in a new class of antidepressive substances called phenylethylamines. These substances are unrelated to TCA and other SSRIs. Venlafaxine blocks the neuronal reuptake of serotonin, noradrenaline, and, to a lesser extent, dopamine in the central nervous system. In contrast with several other antidepressant drugs, venlafaxine can induce a rapid onset of action mainly due to a subsequent norepinephrine reuptake inhibition.[90] See timeline in figure 1.

Society and culture
In 2009, Cymbalta and Effexor were the 11th- and 12th-most-prescribed branded drugs in the United States, respectively. This translates to the 2nd- and 3rd-most-common antidepressants, behind Lexapro (escitalopram), an SSRI.[91]
See also
- Monoamine reuptake inhibitor
- List of antidepressants
- Serotonin releasing agent
- Selective serotonin reuptake inhibitor (SSRI)
- Drug withdrawal
- Serotonin syndrome
- Sexual dysfunction
References
- ↑ "Generic Name Stems - Drug Information Portal - U.S. National Library of Medicine". druginfo.nlm.nih.gov. Archived from the original on 13 April 2021. Retrieved 13 March 2021.
- ↑ The use of stems in the selection of International Nonproprietary Names (INN) for pharmaceutical substances (PDF). WHO. 2011. p. 37. Archived (PDF) from the original on 2018-07-12. Retrieved 2021-04-18.
- ↑ 3.0 3.1 3.2 3.3 3.4 Ansari, Arash; Osser, David. Psychopharmacology: A Concise Overview. Oxford University Press. p. 28. ISBN 978-0-19-753704-6. Archived from the original on 2021-07-10. Retrieved 2021-04-18.
- ↑ 4.0 4.1 4.2 Hitchings, Andrew; Lonsdale, Dagan; Burrage, Daniel; Baker, Emma (2019). The Top 100 Drugs: Clinical Pharmacology and Practical Prescribing (2nd ed.). Elsevier. p. 58. ISBN 978-0-7020-7442-4. Archived from the original on 2021-05-22. Retrieved 2021-11-09.
- ↑ 5.0 5.1 5.2 5.3 5.4 5.5 5.6 "List of SNRIs + Uses, Types & Side Effects". Drugs.com. Archived from the original on 27 April 2021. Retrieved 18 April 2021.
- ↑ 6.0 6.1 Shelton, RC (2019). "Serotonin and Norepinephrine Reuptake Inhibitors". Handbook of experimental pharmacology. 250: 145–180. doi:10.1007/164_2018_164. PMID 30838456.
- ↑ BNF (80 ed.). BMJ Group and the Pharmaceutical Press. September 2020 – March 2021. p. 377. ISBN 978-0-85711-369-6.
{{cite book}}: CS1 maint: date format (link) - ↑ "Venlafaxine Uses, Dosage & Side Effects". Drugs.com. Archived from the original on 9 June 2021. Retrieved 18 April 2021.
- ↑ "The Top 300 of 2021". clincalc.com. Archived from the original on 18 March 2020. Retrieved 18 April 2021.
- ↑ 10.0 10.1 Hillhouse TM, Porter JH (February 2015). "A brief history of the development of antidepressant drugs: from monoamines to glutamate". Experimental and Clinical Psychopharmacology. 23 (1): 1–21. doi:10.1037/a0038550. PMC 4428540. PMID 25643025.
- ↑ "Strattera (atomoxetine) Capsules for Oral Use. Full Prescribing Information" (PDF). Lilly USA, LLC, Indianapolis, IN 46285, USA. Archived (PDF) from the original on 9 July 2014. Retrieved 29 August 2016.
- ↑ Ding YS, Naganawa M, Gallezot JD, Nabulsi N, Lin SF, Ropchan J, et al. (February 2014). "Clinical doses of atomoxetine significantly occupy both norepinephrine and serotonin transports: Implications on treatment of depression and ADHD". NeuroImage. 86: 164–71. doi:10.1016/j.neuroimage.2013.08.001. PMID 23933039.
- ↑ 13.0 13.1 Deecher DC, Beyer CE, Johnston G, Bray J, Shah S, Abou-Gharbia M, Andree TH (August 2006). "Desvenlafaxine succinate: A new serotonin and norepinephrine reuptake inhibitor". The Journal of Pharmacology and Experimental Therapeutics. 318 (2): 657–65. doi:10.1124/jpet.106.103382. PMID 16675639. Archived from the original on 2021-08-29. Retrieved 2019-11-26.
- ↑ 14.0 14.1 Perry R, Cassagnol M (June 2009). "Desvenlafaxine: a new serotonin-norepinephrine reuptake inhibitor for the treatment of adults with major depressive disorder". Clinical Therapeutics. 31 Pt 1 (1): 1374–404. doi:10.1016/j.clinthera.2009.07.012. PMID 19698900.
- ↑ Iyengar S, Webster AA, Hemrick-Luecke SK, Xu JY, Simmons RM (November 2004). "Efficacy of duloxetine, a potent and balanced serotonin-norepinephrine reuptake inhibitor in persistent pain models in rats". The Journal of Pharmacology and Experimental Therapeutics. 311 (2): 576–84. doi:10.1124/jpet.104.070656. PMID 15254142. Archived from the original on 2021-08-29. Retrieved 2019-11-26.
- ↑ 16.0 16.1 16.2 16.3 Hunziker ME, Suehs BT, Bettinger TL, Crismon ML (August 2005). "Duloxetine hydrochloride: a new dual-acting medication for the treatment of major depressive disorder". Clinical Therapeutics. 27 (8): 1126–43. doi:10.1016/j.clinthera.2005.08.010. PMID 16199241.
- ↑ 17.00 17.01 17.02 17.03 17.04 17.05 17.06 17.07 17.08 17.09 17.10 "Cymbalta (duloxetine delayed-release) Capsules for Oral Use. Full Prescribing Information" (PDF). Lilly USA, LLC, Indianapolis, IN 46285, USA. Archived (PDF) from the original on 31 October 2005. Retrieved 29 August 2016.
- ↑ "Yentreve (duloxetine hydrochloride) Hard Gastro-Resistant Capsules. Summary of Product Characteristics" (PDF). European Medicines Agency. Archived (PDF) from the original on 26 June 2016. Retrieved 29 August 2016.
- ↑ "Nefazodone Hydrochloride Tablets. Full Prescribing Information". DailyMed. Teva Pharmaceuticals USA, Inc. North Wales, PA 19454. September 2015. Archived from the original on 16 September 2016. Retrieved 2 September 2016.
- ↑ 20.0 20.1 Frampton JE, Plosker GL (2007). "Duloxetine: a review of its use in the treatment of major depressive disorder". CNS Drugs. 21 (7): 581–609. doi:10.2165/00023210-200721070-00004. PMID 17579500.
- ↑ 21.0 21.1 Morishita S, Arita S (February 2003). "The clinical use of milnacipran for depression". European Psychiatry. 18 (1): 34–5. doi:10.1016/S0924-9338(02)00003-2. PMID 12648895.
- ↑ 22.0 22.1 22.2 22.3 22.4 "Meridia (sibutramine hydrochloride monohydrate) Capsules C-IV. Full Prescribing Information (archived label)". Abbott Laboratories, North Chicago, IL 60064, USA. Archived from the original on 16 September 2016. Retrieved 2 September 2016.
- ↑ Luque CA, Rey JA (April 2002). "The discovery and status of sibutramine as an anti-obesity drug". European Journal of Pharmacology. 440 (2–3): 119–28. doi:10.1016/S0014-2999(02)01423-1. PMID 12007530.
- ↑ Rockoff JD, Dooren JC (October 8, 2010). "Abbott Pulls Diet Drug Meridia Off US Shelves". The Wall Street Journal. Archived from the original on 11 October 2010. Retrieved 8 October 2010.
- ↑ Keating GM (2006). "Tramadol sustained-release capsules". Drugs. 66 (2): 223–30. doi:10.2165/00003495-200666020-00006. PMID 16451094.
- ↑ 26.0 26.1 Gutierrez MA, Stimmel GL, Aiso JY (August 2003). "Venlafaxine: a 2003 update". Clinical Therapeutics. 25 (8): 2138–54. doi:10.1016/s0149-2918(03)80210-2. PMID 14512125.
- ↑ 27.0 27.1 Joffe H, Guthrie KA, LaCroix AZ, Reed SD, Ensrud KE, Manson JE, et al. (July 2014). "Low-dose estradiol and the serotonin-norepinephrine reuptake inhibitor venlafaxine for vasomotor symptoms: a randomized clinical trial". JAMA Internal Medicine. 174 (7): 1058–66. doi:10.1001/jamainternmed.2014.1891. PMC 4179877. PMID 24861828.
- ↑ Redrobe JP, Bourin M, Colombel MC, Baker GB (July 1998). "Dose-dependent noradrenergic and serotonergic properties of venlafaxine in animal models indicative of antidepressant activity". Psychopharmacology. 138 (1): 1–8. doi:10.1007/s002130050638. PMID 9694520.
- ↑ "International OCD Foundation | Medications for OCD". International OCD Foundation. Archived from the original on 2020-05-09. Retrieved 2020-04-18.
- ↑ Publishing, Harvard Health. "Ask Dr. Rob about OCD". Harvard Health. Archived from the original on 2020-04-28. Retrieved 2020-04-18.
- ↑ Sansone, Randy A.; Sansone, Lori A. (2011). "SNRIs Pharmacological Alternatives for the Treatment of Obsessive Compulsive Disorder?". Innovations in Clinical Neuroscience. 8 (6): 10–14. ISSN 2158-8333. PMC 3140892. PMID 21779536.
- ↑ Papakostas GI, Thase ME, Fava M, Nelson JC, Shelton RC (December 2007). "Are antidepressant drugs that combine serotonergic and noradrenergic mechanisms of action more effective than the selective serotonin reuptake inhibitors in treating major depressive disorder? A meta-analysis of studies of newer agents". Biological Psychiatry. 62 (11): 1217–27. doi:10.1016/j.biopsych.2007.03.027. PMID 17588546.
- ↑ Llorca PM, Fernandez JL (April 2007). "Escitalopram in the treatment of major depressive disorder: clinical efficacy, tolerability and cost-effectiveness vs. venlafaxine extended-release formulation". International Journal of Clinical Practice. 61 (4): 702–10. doi:10.1111/j.1742-1241.2007.01335.x. PMID 17394446.
- ↑ 34.0 34.1 Dubovicky, Michal; Belovicova, Kristína; Csatlosova, Kristína; Bogi, Eszter (September 2017). "Risks of using SSRI / SNRI antidepressants during pregnancy and lactation". Interdisciplinary Toxicology. 10 (1): 30–34. doi:10.1515/intox-2017-0004. ISSN 1337-6853. PMC 6096863. PMID 30123033.
- ↑ 35.0 35.1 Strawn, Jeffrey R.; Mills, Jeffrey A.; Sauley, Beau A.; Welge, Jeffrey A. (April 2018). "The Impact of Antidepressant Dose and Class on Treatment Response in Pediatric Anxiety Disorders: A Meta-Analysis". Journal of the American Academy of Child and Adolescent Psychiatry. 57 (4): 235–244.e2. doi:10.1016/j.jaac.2018.01.015. ISSN 0890-8567. PMC 5877120. PMID 29588049.
- ↑ Emslie, Graham J.; Heiligenstein, John H.; Wagner, Karen Dineen; Hoog, Sharon L.; Ernest, Daniel E.; Brown, Eileen; Nilsson, Mary; Jacobson, Jennie G. (October 2002). "Fluoxetine for acute treatment of depression in children and adolescents: a placebo-controlled, randomized clinical trial". Journal of the American Academy of Child and Adolescent Psychiatry. 41 (10): 1205–1215. doi:10.1097/00004583-200210000-00010. ISSN 0890-8567. PMID 12364842. Archived from the original on 2021-08-29. Retrieved 2019-11-26.
- ↑ Mulsant, Benoit H.; Blumberger, Daniel M.; Ismail, Zahinoor; Rabheru, Kiran; Rapoport, Mark J. (August 2014). "A systematic approach to the pharmacotherapy of geriatric major depression". Clinics in Geriatric Medicine. 30 (3): 517–534. doi:10.1016/j.cger.2014.05.002. ISSN 0749-0690. PMC 4122285. PMID 25037293.
- ↑ 38.0 38.1 Boyer EW, Shannon M (March 2005). "The serotonin syndrome". The New England Journal of Medicine. 352 (11): 1112–20. doi:10.1056/NEJMra041867. PMID 15784664. Archived from the original on 2020-10-10. Retrieved 2019-11-26.
- ↑ Zhong, Zhuoyuan; Wang, Limin; Wen, Xiaojun; Liu, Yunyun; Fan, Yafei; Liu, Zhonglin (2017-11-07). "A meta-analysis of effects of selective serotonin reuptake inhibitors on blood pressure in depression treatment: outcomes from placebo and serotonin and noradrenaline reuptake inhibitor controlled trials". Neuropsychiatric Disease and Treatment. 13: 2781–2796. doi:10.2147/NDT.S141832. ISSN 1176-6328. PMC 5683798. PMID 29158677.
- ↑ McIntyre RS et al. The hepatic safety profile of duloxetine: a review. Expert Opin Drug Metab Toxicol. 2008;4(3):281–285.
- ↑ Mladěnka, Přemysl; Applová, Lenka; Patočka, Jiří; Costa, Vera Marisa; Remiao, Fernando; Pourová, Jana; Mladěnka, Aleš; Karlíčková, Jana; Jahodář, Luděk; Vopršalová, Marie; Varner, Kurt J. (July 2018). "Comprehensive review of cardiovascular toxicity of drugs and related agents". Medicinal Research Reviews. 38 (4): 1332–1403. doi:10.1002/med.21476. ISSN 0198-6325. PMC 6033155. PMID 29315692.
- ↑ 42.0 42.1 42.2 42.3 42.4 "Savella (milnacipran HCl) Tablets. Full Prescribing Information". Allergan USA, Inc. Irvine, CA 92612. August 2016. Archived from the original on 29 August 2016. Retrieved 2 September 2016.
- ↑ "SNRI Antidepressants". poison.org. Archived from the original on 2020-08-07. Retrieved 2019-10-21.
- ↑ Versiani M, Cassano G, Perugi G, Benedetti A, Mastalli L, Nardi A, Savino M (January 2002). "Reboxetine, a selective norepinephrine reuptake inhibitor, is an effective and well-tolerated treatment for panic disorder". The Journal of Clinical Psychiatry. 63 (1): 31–7. doi:10.4088/jcp.v63n0107. PMID 11838623.
- ↑ 45.0 45.1 45.2 45.3 "Pristiq (desvenlafaxine) Extended-Release Tablets, for Oral Use. Full Prescribing Information". Wyeth Pharmaceuticals, Inc. A subsidiary of Pfizer, Inc. Philadelphia, PA 19101. July 2016. Archived from the original on 14 August 2016. Retrieved 2 September 2016.
- ↑ 46.0 46.1 46.2 46.3 "Effexor XR (venlafaxine) Extended-Release Capsules. Full Prescribing Information". Wyeth Pharmaceuticals, Inc. A subsidiary of Pfizer, Inc. Philadelphia, PA 19101. August 2015. Archived from the original on 14 August 2012. Retrieved 2 September 2016.
- ↑ "Cymbalta Package Insert" (PDF). Archived (PDF) from the original on 2019-11-07. Retrieved 2019-11-07.
- ↑ "Effexor XR Prescribing Information". Archived from the original on 2012-08-14. Retrieved 2012-07-27.
- ↑ Olivier JD, Olivier B (2019-09-01). "Antidepressants and Sexual Dysfunctions: a Translational Perspective". Current Sexual Health Reports. 11 (3): 156–166. doi:10.1007/s11930-019-00205-y. ISSN 1548-3592.
- ↑ Clayton AH, Montejo AL (2006). "Major depressive disorder, antidepressants, and sexual dysfunction". The Journal of Clinical Psychiatry. 67 Suppl 6: 33–7. PMID 16848675.
- ↑ 51.0 51.1 Jing E, Straw-Wilson K (July 2016). "Sexual dysfunction in selective serotonin reuptake inhibitors (SSRIs) and potential solutions: A narrative literature review". The Mental Health Clinician. 6 (4): 191–196. doi:10.9740/mhc.2016.07.191. PMC 6007725. PMID 29955469.
- ↑ "Serotonin syndrome: Preventing, recognizing, and treating it". www.mdedge.com. Archived from the original on 2019-10-11. Retrieved 2019-11-21.
- ↑ 53.0 53.1 Frank C (July 2008). "Recognition and treatment of serotonin syndrome". Canadian Family Physician. 54 (7): 988–92. PMC 2464814. PMID 18625822.
- ↑ "SNRI Antidepressants". poison.org. Archived from the original on 2020-08-07. Retrieved 2019-10-23.
- ↑ Tint, Aung; Haddad, Peter M; Anderson, Ian M (2008-05-01). "The effect of rate of antidepressant tapering on the incidence of discontinuation symptoms: a randomised study". Journal of Psychopharmacology. 22 (3): 330–332. doi:10.1177/0269881107081550. ISSN 0269-8811. PMID 18515448. Archived from the original on 2021-08-29. Retrieved 2019-11-26.
- ↑ 56.0 56.1 Cochran KA, Cavallari LH, Shapiro NL, Bishop JR (August 2011). "Bleeding incidence with concomitant use of antidepressants and warfarin". Therapeutic Drug Monitoring. 33 (4): 433–8. doi:10.1097/FTD.0b013e318224996e. PMC 3212440. PMID 21743381.
- ↑ Cheng YL, Hu HY, Lin XH, Luo JC, Peng YL, Hou MC, et al. (November 2015). "Use of SSRI, But Not SNRI, Increased Upper and Lower Gastrointestinal Bleeding: A Nationwide Population-Based Cohort Study in Taiwan". Medicine. 94 (46): e2022. doi:10.1097/MD.0000000000002022. PMC 4652818. PMID 26579809.
- ↑ 58.0 58.1 Zeind, Caroline; Carvalho (2018). Applied Therapeutics: The Clinical Use of Drugs, 11e. Wolters Kluwer. pp. 1813–1833. ISBN 9781496318299.
- ↑ "Duloxetine: Drug Information". UpToDate. Archived from the original on 22 February 2014. Retrieved 28 June 2012.
- ↑ Perahia DG, Pritchett YL, Kajdasz DK, Bauer M, Jain R, Russell JM, et al. (January 2008). "A randomized, double-blind comparison of duloxetine and venlafaxine in the treatment of patients with major depressive disorder". Journal of Psychiatric Research. 42 (1): 22–34. doi:10.1016/j.jpsychires.2007.01.008. PMID 17445831.
- ↑ Wilson E, Lader M (December 2015). "A review of the management of antidepressant discontinuation symptoms". Therapeutic Advances in Psychopharmacology. 5 (6): 357–68. doi:10.1177/2045125315612334. PMC 4722507. PMID 26834969.
- ↑ Fava GA, Benasi G, Lucente M, Offidani E, Cosci F, Guidi J (2018). "Withdrawal Symptoms after Serotonin-Noradrenaline Reuptake Inhibitor Discontinuation: Systematic Review" (PDF). Psychotherapy and Psychosomatics. 87 (4): 195–203. doi:10.1159/000491524. PMID 30016772.
- ↑ Fava, Giovanni A.; Benasi, Giada; Lucente, Marcella; Offidani, Emanuela; Cosci, Fiammetta; Guidi, Jenny (2018). "Withdrawal Symptoms after Serotonin-Noradrenaline Reuptake Inhibitor Discontinuation: Systematic Review". Psychotherapy and Psychosomatics. 87 (4): 195–203. doi:10.1159/000491524. hdl:2158/1132671. ISSN 0033-3190. PMID 30016772.
- ↑ Taylor D, Lenox-Smith A, Bradley A (June 2013). "A review of the suitability of duloxetine and venlafaxine for use in patients with depression in primary care with a focus on cardiovascular safety, suicide and mortality due to antidepressant overdose". Therapeutic Advances in Psychopharmacology. 3 (3): 151–61. doi:10.1177/2045125312472890. PMC 3805457. PMID 24167687.
- ↑ Simon, Leslie V.; Hashmi, Muhammad F.; Keenaghan, Michael (2019), "Serotonin Syndrome", StatPearls, StatPearls Publishing, PMID 29493999, archived from the original on 2020-10-25, retrieved 2019-11-21
- ↑ Troy, S. M.; Parker, V. P.; Hicks, D. R.; Pollack, G. M.; Chiang, S. T. (October 1997). "Pharmacokinetics and effect of food on the bioavailability of orally administered venlafaxine". Journal of Clinical Pharmacology. 37 (10): 954–961. doi:10.1002/j.1552-4604.1997.tb04270.x. ISSN 0091-2700. PMID 9505987.
- ↑ Wichniak A, Wierzbicka A, Walęcka M, Jernajczyk W (August 2017). "Effects of Antidepressants on Sleep". Current Psychiatry Reports. 19 (9): 63. doi:10.1007/s11920-017-0816-4. PMC 5548844. PMID 28791566.
- ↑ "Helpful for chronic pain in addition to depression". Mayo Clinic. Archived from the original on 2019-10-21. Retrieved 2019-10-24.
- ↑ 69.0 69.1 Grandoso L, Pineda J, Ugedo L (May 2004). "Comparative study of the effects of desipramine and reboxetine on locus coeruleus neurons in rat brain slices". Neuropharmacology. 46 (6): 815–23. doi:10.1016/j.neuropharm.2003.11.033. PMID 15033341.
- ↑ Brunello N, Mendlewicz J, Kasper S, Leonard B, Montgomery S, Nelson J, et al. (October 2002). "The role of noradrenaline and selective noradrenaline reuptake inhibition in depression" (PDF). European Neuropsychopharmacology. 12 (5): 461–75. doi:10.1016/s0924-977x(02)00057-3. PMID 12208564. Archived from the original on 2021-08-29. Retrieved 2020-03-20.
- ↑ Stahl SM, Grady MM, Moret C, Briley M (September 2005). "SNRIs: their pharmacology, clinical efficacy, and tolerability in comparison with other classes of antidepressants". CNS Spectrums. 10 (9): 732–47. doi:10.1017/S1092852900019726. PMID 16142213.
- ↑ Santarsieri, Daniel; Schwartz, Thomas L (2015-10-08). "Antidepressant efficacy and side-effect burden: a quick guide for clinicians". Drugs in Context. 4: 212290. doi:10.7573/dic.212290. ISSN 1745-1981. PMC 4630974. PMID 26576188.
- ↑ Clevenger, Steven S.; Malhotra, Devvrat; Dang, Jonathan; Vanle, Brigitte; IsHak, Waguih William (2018). "The role of selective serotonin reuptake inhibitors in preventing relapse of major depressive disorder". Therapeutic Advances in Psychopharmacology. 8 (1): 49–58. doi:10.1177/2045125317737264. ISSN 2045-1253. PMC 5761909. PMID 29344343.
- ↑ 74.0 74.1 Lemke TL, Williams DA, Roche VF, Zito SW (2008). Foye´s principles of medicinal chemistry (6th ed.). USA: Lippincott Williams & Wilkins. pp. 547–67, 581–582.
- ↑ 75.0 75.1 75.2 75.3 Boot J, Cases M, Clark BP, Findlay J, Gallagher PT, Hayhurst L, et al. (February 2005). "Discovery and structure-activity relationships of novel selective norepinephrine and dual serotonin/norepinephrine reuptake inhibitors". Bioorganic & Medicinal Chemistry Letters. 15 (3): 699–703. doi:10.1016/j.bmcl.2004.11.025. PMID 15664840.
- ↑ Nutt DJ (2008). "Relationship of neurotransmitters to the symptoms of major depressive disorder". The Journal of Clinical Psychiatry. 69 Suppl E1: 4–7. PMID 18494537.
- ↑ Sindrup SH, Otto M, Finnerup NB, Jensen TS (June 2005). "Antidepressants in the treatment of neuropathic pain". Basic & Clinical Pharmacology & Toxicology. 96 (6): 399–409. doi:10.1111/j.1742-7843.2005.pto_96696601.x. PMID 15910402.
- ↑ Shelton RC, Miller AH (2011). "Inflammation in depression: is adiposity a cause?". Dialogues in Clinical Neuroscience. 13 (1): 41–53. PMC 3181969. PMID 21485745.
- ↑ 79.0 79.1 Tynan RJ, Weidenhofer J, Hinwood M, Cairns MJ, Day TA, Walker FR (March 2012). "A comparative examination of the anti-inflammatory effects of SSRI and SNRI antidepressants on LPS stimulated microglia". Brain, Behavior, and Immunity. 26 (3): 469–79. doi:10.1016/j.bbi.2011.12.011. PMID 22251606.
- ↑ "Archive copy". Archived from the original on 2016-08-10. Retrieved 2016-02-07.
{{cite web}}: CS1 maint: archived copy as title (link) - ↑ Lambert O, Bourin M (November 2002). "SNRIs: mechanism of action and clinical features". Expert Review of Neurotherapeutics. 2 (6): 849–58. doi:10.1586/14737175.2.6.849. PMID 19810918.
- ↑ 82.0 82.1 Raouf M, Glogowski AJ, Bettinger JJ, Fudin J (August 2017). "Serotonin-norepinephrine reuptake inhibitors and the influence of binding affinity (Ki) on analgesia". Journal of Clinical Pharmacy and Therapeutics. 42 (4): 513–517. doi:10.1111/jcpt.12534. PMID 28503727.
- ↑ 83.0 83.1 83.2 Upadhyaya HP, Desaiah D, Schuh KJ, Bymaster FP, Kallman MJ, Clarke DO, et al. (March 2013). "A review of the abuse potential assessment of atomoxetine: a nonstimulant medication for attention-deficit/hyperactivity disorder". Psychopharmacology. Springer Nature. 226 (2): 189–200. doi:10.1007/s00213-013-2986-z. PMC 3579642. PMID 23397050.
- ↑ Roth BL, Driscol J (Dec 2012). "PDSP Ki Database". Psychoactive Drug Screening Program (PDSP). University of North Carolina at Chapel Hill and the United States National Institute of Mental Health. Archived from the original on 28 August 2021. Retrieved 7 July 2018.
- ↑ 85.0 85.1 85.2 Mahaney PE, Vu AT, McComas CC, Zhang P, Nogle LM, Watts WL, et al. (December 2006). "Synthesis and activity of a new class of dual acting norepinephrine and serotonin reuptake inhibitors: 3-(1H-indol-1-yl)-3-arylpropan-1-amines". Bioorganic & Medicinal Chemistry. 14 (24): 8455–66. doi:10.1016/j.bmc.2006.08.039. PMID 16973367.
- ↑ Mahaney PE, Gavrin LK, Trybulski EJ, Stack GP, Vu TA, Cohn ST, et al. (July 2008). "Structure-activity relationships of the cycloalkanol ethylamine scaffold: discovery of selective norepinephrine reuptake inhibitors". Journal of Medicinal Chemistry. 51 (13): 4038–49. doi:10.1021/jm8002262. PMID 18557608.
- ↑ 87.0 87.1 87.2 Chen C, Dyck B, Fleck BA, Foster AC, Grey J, Jovic F, et al. (February 2008). "Studies on the SAR and pharmacophore of milnacipran derivatives as monoamine transporter inhibitors". Bioorganic & Medicinal Chemistry Letters. 18 (4): 1346–9. doi:10.1016/j.bmcl.2008.01.011. PMID 18207394.
- ↑ 88.0 88.1 88.2 Tamiya J, Dyck B, Zhang M, Phan K, Fleck BA, Aparicio A, et al. (June 2008). "Identification of 1S,2R-milnacipran analogs as potent norepinephrine and serotonin transporter inhibitors". Bioorganic & Medicinal Chemistry Letters. 18 (11): 3328–32. doi:10.1016/j.bmcl.2008.04.025. PMID 18445525.
- ↑ 89.0 89.1 Lieberman JA (2003). "History of the Use of Antidepressants in Primary Care" (PDF). Primary Care Companion J Clin Psychiatry. 5 (S7): 6–10. Archived (PDF) from the original on 2014-06-11. Retrieved 2014-03-05.
- ↑ Ruelas EG, Diaz-Martinez A, Ruiz RM, Study TV, Group C (1997). "An open assessment of the acceptability, efficacy, and tolerance of venlafaxine in usual care settings". Current Therapeutic Research. 58 (9): 609–630. doi:10.1016/S0011-393X(97)80088-4.
- ↑ "2009 Top 200 branded drugs by total prescriptions" (PDF). SDI/Verispan, VONA, full year 2009. www.drugtopics.com. Archived from the original (PDF) on 14 July 2011. Retrieved 6 April 2011.
External links
- Pages using duplicate arguments in template calls
- CS1 maint: date format
- CS1: long volume value
- CS1 maint: archived copy as title
- Articles that may contain original research from June 2017
- Articles with invalid date parameter in template
- Articles with hatnote templates targeting a nonexistent page
- Articles using infobox templates with no data rows
- Serotonin-norepinephrine reuptake inhibitors
- RTT