Lasmiditan
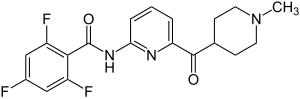 | |
| Names | |
|---|---|
| Trade names | Reyvow |
| Other names | COL-144 |
| |
| Clinical data | |
| Drug class | 5-HT1F receptor activator[1] |
| Main uses | Migraine headaches[2] |
| Side effects | Dizziness, tiredness, numbness[1] |
| Routes of use | By mouth |
| Typical dose | 50 to 200 mg[2] |
| External links | |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a620015 |
| Legal | |
| License data |
|
| Legal status |
|
| Chemical and physical data | |
| Formula | C19H18F3N3O2 |
| Molar mass | 377.367 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Lasmiditan, sold under the brand name Reyvow, is a medication used to treat migraine headaches.[2] It is not useful for prevention.[2] It is taken by mouth.[2] It is less effective and more expensive than sumatriptan.[4]
Common side effects include dizziness, tiredness, and numbness.[1] Other side effects may include serotonin syndrome, medication overuse headaches, and poor coordination.[1] It has a low risk for misuse.[2] Safety in pregnancy is unclear.[2] It is a 5-HT1F receptor activator.[1]
Lasmiditan was approved for medical use in the United States in 2019.[2] In the United States it costs about 83 USD per 100 mg dose as of 2021.[5] It is not available in the United Kingdom or Europe as of 2021.[4]
Medical uses
Dosage
It is taken at a dose of 50 to 200 mg.[2] It should not be used more than once per day.[1]
Lasmiditan is delivered in 50 and 100 mg tablets.[6]
Side effects
There is a risk of driving impairment while taking lasmiditan. People are advised not to drive or operate machinery for at least eight hours after taking lasmiditan, even if they feel well enough to do so. People who cannot follow this advice are advised not to take lasmiditan. The drug causes central nervous system (CNS) depression, including dizziness and sedation. It should be used with caution if taken in combination with alcohol or other CNS depressants.[1]
Mechanism of action
Lasmiditan is a serotonin receptor agonist that, like the unsuccessful LY-334,370, selectively binds to the 5-HT1F receptor subtype. A number of triptans have been shown to act on this subtype as well, but only after their affinity for 5-HT1B and 5-HT1D has been made responsible for their anti-migraine activity. The lack of affinity for these receptors might result in fewer side effects related to vasoconstriction compared to triptans in susceptible people, such as those with ischemic heart disease, Raynaud's phenomenon or after a myocardial infarction,[7] although a 1998 review has found such side-effects to rarely occur in people taking triptans.[8][9]
History
Lasmiditan was discovered by Eli Lilly and Company and was then relicensed to CoLucid Pharmaceuticals in 2006, until CoLucid was bought by Eli Lilly in 2017, to allow Eli Lilly to reacquire the drug's intellectual property.[10] The drug is protected by patents until 2031.[11]
Eli Lilly submitted a new drug application to the U.S. Food and Drug Administration (FDA) in November 2018.[12]
The FDA approved the drug in October 2019.[13] However, as of October 2019[update], the drug was awaiting Drug Enforcement Administration (DEA) scheduling before it was made available in the United States.[14] It was placed into Schedule V in January 2020.[15][3]
The U.S. Food and Drug Administration (FDA) considers it to be a first-in-class medication.[16]
Research
Phase II clinical trials for dose finding purposes were completed in 2007, for an intravenous form[17] and in early 2010, for an oral form.[18]
Three Phase III clinical trials were completed. The SPARTAN trial compared placebo with 50, 100, and 200 mg of lasmiditan.[19] SAMURAI compared placebo with 100 and 200 mg doses of lasmiditan. GLADIATOR is an open-label study that compared 100 and 200 mg doses of lasmiditan in subjects that received the drug as part of a prior trial.[20]
Topline results from the SPARTAN trial showed that the drug induced met its primary and secondary endpoints in the trial. The primary result showed a statistically significant improvement in pain relief relative to placebo 2 hours after the first dose. The secondary result showed a statistically significantly greater percentage of subjects were free of their most bothersome symptom (MBS) compared with placebo at two hours following the first dose.[21]
The FDA approved lasmiditan primarily based on data from two clinical trials, Trial 1 (# NCT02439320) and Trial 2 (#NCT02605174) of 4439 subjects with migraine headaches with or without aura.[13] Trials were conducted at 224 sites in the United States, the United Kingdom, and Germany.[13]
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 1.6 "Reyvow- lasmiditan tablet". DailyMed. 11 October 2019. Archived from the original on 25 November 2020. Retrieved 15 November 2019.
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 2.6 2.7 2.8 "Lasmiditan Monograph for Professionals". Drugs.com. Archived from the original on 23 January 2021. Retrieved 21 November 2021.
- ↑ 3.0 3.1 "2020 - Placement of Lasmiditan in Schedule V". DEA Diversion Control Division. 31 January 2020. Archived from the original on 31 January 2020. Retrieved 31 January 2020.
- ↑ 4.0 4.1 "Lasmiditan". SPS - Specialist Pharmacy Service. 30 December 2015. Archived from the original on 21 November 2021. Retrieved 21 November 2021.
- ↑ "Reyvow Prices and Reyvow Coupons - GoodRx". GoodRx. Retrieved 21 November 2021.
- ↑ "Reyvow (Lasmiditan Tablets): Uses, Dosage, Side Effects, Interactions, Warning". RxList. Archived from the original on 25 January 2021. Retrieved 20 August 2020.
- ↑ "Molecule of the Month July 2010: Lasmiditan hydrochloride". Prous Science. Archived from the original on 28 July 2011. Retrieved 3 August 2011.
- ↑ Dahlöf CG, Mathew N (October 1998). "Cardiovascular safety of 5HT1B/1D agonists--is there a cause for concern?". Cephalalgia. 18 (8): 539–45. doi:10.1046/j.1468-2982.1998.1808539.x. PMID 9827245. S2CID 30125923.
- ↑ Mutschler E, Geisslinger G, Kroemer HK, Schäfer-Korting M (2001). Arzneimittelwirkungen (in German) (8th ed.). Stuttgart: Wissenschaftliche Verlagsgesellschaft. p. 265. ISBN 978-3-8047-1763-3. OCLC 47700647.
{{cite book}}: CS1 maint: unrecognized language (link) - ↑ "Lilly buys migraine biotech CoLucid, and the drug it outlicensed, for $960M". Archived from the original on 25 September 2020. Retrieved 24 October 2021.
- ↑ "Lasmiditan - Eli Lilly and Company - AdisInsight". Archived from the original on 26 September 2021. Retrieved 24 October 2021.
- ↑ "Lilly Submits New Drug Application to the FDA for Lasmiditan for Acute Treatment of Migraine, Receives Breakthrough Therapy Designation for Emgality (galcanezumab-gnlm) for Prevention of Episodic Cluster Headache". Eli Lilly and Company. 14 November 2018. Archived from the original on 12 October 2019. Retrieved 12 October 2019 – via PR Newswire.
- ↑ 13.0 13.1 13.2 "Drug Trials Snapshots: Reyvow". U.S. Food and Drug Administration (FDA). 11 October 2019. Archived from the original on 13 December 2019. Retrieved 26 January 2020.
- ↑ Vinluan F (11 October 2019). "FDA OKs Lilly's Lasmiditan, First New Acute Migraine Drug in Decades". Xconomy. Archived from the original on 12 October 2019. Retrieved 12 October 2019.
- ↑ "Schedules of Controlled Substances: Placement of Lasmiditan in Schedule V". Federal Register. 31 January 2020. Archived from the original on 26 March 2021. Retrieved 24 October 2021.
- ↑ "New Drug Therapy Approvals 2019". U.S. Food and Drug Administration. 31 December 2019. Archived from the original on 16 September 2020. Retrieved 15 September 2020.
- ↑ "A Placebo-Controlled Adaptive Treatment Assignment Study of Intravenous COL-144 in the Acute Treatment of Migraine". ClinicalTrials.gov. 8 November 2019. Archived from the original on 23 February 2020. Retrieved 23 February 2020.
- ↑ "Dose-ranging Study of Oral COL-144 in Acute Migraine Treatment". ClinicalTrials.gov. 20 December 2019. Archived from the original on 23 February 2020. Retrieved 23 February 2020.
- ↑ Clinical trial number NCT02605174 for "Three Doses of Lasmiditan (50 mg, 100 mg and 200 mg) Compared to Placebo in the Acute Treatment of Migraine (SPARTAN)" at ClinicalTrials.gov
- ↑ Clinical trial number NCT02565186 for "An Open-label, Long-term, Safety Study of Lasmiditan for the Acute Treatment of Migraine (GLADIATOR)" at ClinicalTrials.gov
- ↑ "Lilly Announces Positive Results for Second Phase 3 Study of Lasmiditan for the Acute Treatment of Migraine". Archived from the original on 5 August 2017. Retrieved 5 August 2017.
External links
| External sites: | |
|---|---|
| Identifiers: |
|
- Pages using duplicate arguments in template calls
- CS1 maint: unrecognized language
- Use dmy dates from November 2019
- Articles with invalid date parameter in template
- Articles with changed InChI identifier
- Chemical articles with unknown parameter in Infobox drug
- Chemical articles without CAS registry number
- Articles without EBI source
- Chemical pages without ChemSpiderID
- Chemical pages without DrugBank identifier
- Articles without KEGG source
- Articles without UNII source
- Drugs missing an ATC code
- Drugboxes which contain changes to verified fields
- Articles containing potentially dated statements from October 2019
- All articles containing potentially dated statements
- Drugs not assigned an ATC code
- Articles with changed CASNo identifier
- Articles with changed ChemSpider identifier
- Articles with changed KEGG identifier
- 5-HT1F receptor agonists
- Antimigraine drugs
- Benzamides
- Eli Lilly and Company brands
- Fluoroarenes
- Ketones
- Piperidines
- Pyridines
- RTT