Oxymetazoline
 | |
| Names | |
|---|---|
| Trade names | Afrin, Ocuclear, Drixine, others |
| Other names | Oxymetazoline hydrochloride |
| |
| Clinical data | |
| Drug class | Alpha adrenergic agonist[1] |
| Main uses | Stuffy nose, eye redness[1] |
| Side effects | Burning, nasal discharge[1] |
| Dependence risk | Moderate |
| Pregnancy category |
|
| Routes of use | Intranasal, eye drops |
| Onset of action | Within 10 min[1] |
| Duration of action | 6 hrs[1] |
| External links | |
| AHFS/Drugs.com | General: Monograph Topical: Monograph |
| Legal | |
| Legal status |
|
| Pharmacokinetics | |
| Metabolism | Kidney (30%), fecal (10%) |
| Elimination half-life | 5–6 hours |
| Chemical and physical data | |
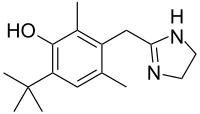
| Formula | C16H24N2O |
| Molar mass | 260.381 g·mol−1 |
| 3D model (JSmol) | |
| Melting point | 301.5 °C (574.7 °F) |
| |
| |
Oxymetazoline, sold under many brand name, is a medication used to treat a stuffy nose or eye redness due to minor irritation.[1] It is available as a nasal spray or eye drops.[1] Benefits are seen within 10 minutes and last for up to 6 hours.[1] No more than 3 to 5 days of use is recommended; with use recommended against in those under 6 years.[2][3]
Common side effects may include burning and nasal discharge.[1] Other side effects may include recurrence of stuffiness following stopping use, headache, palpitations, and nervousness.[1] Safety in pregnancy is unclear.[1] It works by activating alpha adrenergic receptor which cases small arteries to narrow.[1]
Oxymetazoline was first made in 1961 and was approved for medical use in the United States in 1986.[2][1] It is available as a generic medication and over the counter.[1] In the United States 30 ml of solution costs about 12 USD as of 2021.[4]
Medical uses
It is used to treat persistent facial redness associated with rosacea in adults.[5]
Due to its vasoconstricting properties, oxymetazoline is also used to treat nose bleeds[6][7] and eye redness due to minor irritation.
It may also be used for acquired drooping eyelid.[8]
It may also have benefit in fecal incontinence due to spinal cord injury.[9]
Side effects
Rebound congestion
Rebound congestion, or rhinitis medicamentosa, may occur. A 2006 review of the pathology of rhinitis medicamentosa concluded that use of oxymetazoline for more than three days may result in rhinitis medicamentosa and recommended limiting use to three days.[10] In a submission to the Therapeutic Goods Administration, a Novartis representative concluded, "The justification was not based on evidence." Citing an existing extensive body of evidence and noting a range of recommended periods from five to ten days, Novartis recommended the established five day period for its use for self-medication without medical consultation as it coincides with the typical duration of the common cold.[11]
Pregnancy
The Food and Drug Administration places oxymetazoline in category C, indicating risk to the fetus cannot be ruled out. While it has been shown that a single dose does not significantly alter either maternal or fetal circulation,[12] this subject has not been studied extensively enough to draw reliable conclusions.[original research?]
Overdose
If accidentally ingested, standard methods to remove unabsorbed drugs should be considered.[clarification needed] There is no specific antidote for oxymetazoline, although its pharmacological effects may be reversed by α adrenergic antagonists such as phentolamine. In the event of a possibly life-threatening overdose (such as a hypertensive crisis), benzodiazepines should be considered to decrease the likelihood of seizures and convulsions, as well as reduce anxiety and to lower blood pressure. In children, oxymetazoline may produce profound central nervous system depression due to stimulation of central α2 receptors and imidazoline receptors, much like clonidine.[citation needed]
Pharmacology
Pharmacodynamics
Oxymetazoline is a sympathomimetic that selectively agonizes α1 and, partially, α2 adrenergic receptors.[13] Since vascular beds widely express α1 receptors, the action of oxymetazoline results in vasoconstriction. In addition, the local application of the drug also results in vasoconstriction due to its action on endothelial postsynaptic α2 receptors; systemic application of α2 agonists, in contrast, causes vasodilation because of centrally-mediated inhibition of sympathetic tone via presynaptic α2 receptors.[14] Vasoconstriction of vessels results in relief of nasal congestion in two ways: first, it increases the diameter of the airway lumen; second, it reduces fluid exudation from postcapillary venules.[15] It can reduce nasal airway resistance (NAR) up to 35.7% and reduce nasal mucosal blood flow up to 50%.[16]
Pharmacokinetics
Imidazolines are sympathomimetic agents, with primary effects on α adrenergic receptors and little if any effect on β adrenergic receptors.[citation needed] Oxymetazoline is readily absorbed orally.[citation needed] Effects on α receptors from systemically absorbed oxymetazoline hydrochloride may persist for up to 7 hours after a single dose.[citation needed] The elimination half-life in humans is 5–8 hours.[citation needed] It is excreted unchanged both by the kidneys (30%) and in feces (10%).[citation needed]
History
Oxymetazoline was developed from xylometazoline at E. Merck Darmstadt by Wolfgang Fruhstorfer and Helmut Müller-Calgan in 1961.[17]
The oxymetazoline brand Afrin was first sold as a prescription medication in 1966. After finding substantial early success as a prescription medication, it became available as an over-the-counter drug in 1975. Schering-Plough did not engage in heavy advertising until 1986.[18]
From the mid 1980s to mid 1990s, the brand Sinex was featured in many television advertisements. Some of these commercials showed men, women, and children using other brands of nasal sprays, and then standing upside down on a sidewalk, or against a wall, etc or hanging upside down from various playground equipment to prevent their nasal spray from dripping out. This was juxtaposed with Sinex users not having to hang upside side down or stand on their heads as it didn't drip out.
Society and culture
A generic version of the cream was first approved in 2021 in the USA.[19]
Brand names
Brand names include Afrin, Drixine, Dristan, Nasivin, Nasivion, Nezeril, Nostrilla, Logicin, Vicks Sinex, Visine L.R., Sudafed OM, Zicam, Otrivin Oxy, SinuFrin, Upneeq, and Mucinex Sinus-Max.
Otrivin, Afrin, Operil, Dristan, Dimetapp, Oxyspray, Facimin, Nasivin, Nostrilla, Utabon, Sudafed OM, Vicks Sinex, Zicam, SinuFrin, Drixoral[20] and Mucinex Full Force.[21]
References
- ↑ 1.00 1.01 1.02 1.03 1.04 1.05 1.06 1.07 1.08 1.09 1.10 1.11 1.12 1.13 "Oxymetazoline Monograph for Professionals". Drugs.com. Archived from the original on 12 August 2021. Retrieved 10 November 2021.
- ↑ 2.0 2.1 Velpandian, Thirumurthy (29 February 2016). Pharmacology of Ocular Therapeutics. Springer. p. 172. ISBN 978-3-319-25498-2.
- ↑ BNF 81: March-September 2021. BMJ Group and the Pharmaceutical Press. 2021. p. 1247. ISBN 978-0857114105.
- ↑ "Oxymetazoline nasal Prices, Coupons & Patient Assistance Programs". Drugs.com. Archived from the original on 19 January 2021. Retrieved 10 November 2021.
- ↑ Patel NU, Shukla S, Zaki J, Feldman SR (October 2017). "Oxymetazoline hydrochloride cream for facial erythema associated with rosacea". Expert Review of Clinical Pharmacology. 10 (10): 1049–1054. doi:10.1080/17512433.2017.1370370. PMID 28837365. S2CID 19930755.
- ↑ Katz RI, Hovagim AR, Finkelstein HS, Grinberg Y, Boccio RV, Poppers PJ (1990). "A comparison of cocaine, lidocaine with epinephrine, and oxymetazoline for prevention of epistaxis on nasotracheal intubation". Journal of Clinical Anesthesia. 2 (1): 16–20. doi:10.1016/0952-8180(90)90043-3. PMID 2310576.
- ↑ Krempl GA, Noorily AD (September 1995). "Use of oxymetazoline in the management of epistaxis". The Annals of Otology, Rhinology, and Laryngology. 104 (9 Pt 1): 704–6. doi:10.1177/000348949510400906. PMID 7661519. S2CID 37579139.
- ↑ "UPNEEQ® Label" (PDF). accessdata.fda.gov. 8 July 2020. Archived (PDF) from the original on 2 April 2021. Retrieved 16 June 2021.
- ↑ "EU/3/17/1892: Orphan designation for the treatment of spinal cord injury". Archived from the original on 10 November 2021. Retrieved 10 November 2021.
- ↑ Ramey JT, Bailen E, Lockey RF (2006). "Rhinitis medicamentosa". Journal of Investigational Allergology & Clinical Immunology. 16 (3): 148–55. PMID 16784007. Archived from the original on 23 October 2020. Retrieved 16 June 2021.
- ↑ Nguyen, Tra-My (2014). "Consultation submission: OTC nasal decongestant preparations for topical use: proposed advisory statements for medicines" (PDF). Novartis Consumer Health Australasia. Archived (PDF) from the original on 2 August 2018. Retrieved 16 June 2021.
- ↑ Rayburn WF, Anderson JC, Smith CV, Appel LL, Davis SA (August 1990). "Uterine and fetal Doppler flow changes from a single dose of a long-acting intranasal decongestant". Obstetrics and Gynecology. 76 (2): 180–2. PMID 2196495. Archived from the original on 2 September 2019. Retrieved 16 June 2021.
- ↑ Westfall Thomas C, Westfall David P, "Chapter 6. Neurotransmission: The Autonomic and Somatic Motor Nervous Systems" (Chapter). Brunton LL, Lazo JS, Parker KL: Goodman & Gilman's The Pharmacological Basis of Therapeutics, 11e: "Archived copy". Archived from the original on 30 September 2011. Retrieved 24 January 2015.
{{cite web}}: CS1 maint: archived copy as title (link). - ↑ Biaggioni I, Robertson D. "Chapter 9. Adrenoceptor Agonists & Sympathomimetic Drugs". In Katzung BG (ed.). Basic & Clinical Pharmacology (11th ed.). Archived from the original on 30 September 2011. Retrieved 30 November 2011.
- ↑ Widdicombe J (1997). "Microvascular anatomy of the nose". Allergy. 52 (40 Suppl): 7–11. doi:10.1111/j.1398-9995.1997.tb04877.x. PMID 9353554. S2CID 46018611.
- ↑ Bende M, Löth S (March 1986). "Vascular effects of topical oxymetazoline on human nasal mucosa". The Journal of Laryngology and Otology. 100 (3): 285–8. doi:10.1017/S0022215100099151. PMID 3950497.
- ↑ German Patent 1,117,588
- ↑ Dougherty, Phillip H. (20 October 1986). "Advertising; Afrin Goes After Users Of Nasal Decongestants". The New York Times. Archived from the original on 2 September 2019. Retrieved 30 March 2015.
- ↑ Research, Center for Drug Evaluation and (10 February 2022). "2021 First Generic Drug Approvals". FDA. Archived from the original on 21 June 2022. Retrieved 22 October 2022.
- ↑ "Drixoral Decongestant Nasal Spray". Bayer. Archived from the original on 1 December 2017. Retrieved 18 November 2017.
- ↑ "Oxymetazoline: Drug Information Provided by Lexi-Comp: Merck Manual Professional". Merck.com. Archived from the original on 10 April 2010. Retrieved 15 April 2013.
External links
| Identifiers: |
|
|---|
- Pages using duplicate arguments in template calls
- CS1 maint: archived copy as title
- Use dmy dates from September 2018
- Articles with invalid date parameter in template
- Drugs with non-standard legal status
- Chemical articles with unknown parameter in Infobox drug
- Chemical articles without CAS registry number
- Articles without EBI source
- Chemical pages without ChemSpiderID
- Chemical pages without DrugBank identifier
- Articles without KEGG source
- Articles without UNII source
- Drugs missing an ATC code
- Drugboxes which contain changes to verified fields
- Drugboxes which contain changes to watched fields
- All articles that may contain original research
- Articles that may contain original research from September 2018
- Wikipedia articles needing clarification from December 2016
- All articles with unsourced statements
- Articles with unsourced statements from October 2011
- Articles with unsourced statements from September 2018
- Alpha-adrenergic agonists
- Imidazolines
- Phenols
- Schering-Plough brands
- Merck & Co. brands
- Bayer brands
- Topical decongestants
- Vasoconstrictors
- Tert-butyl compounds
- RTT