Prucalopride
 | |
| Names | |
|---|---|
| Trade names | Prudac, Resolor, Resotran, other |
| |
| Clinical data | |
| Drug class | 5-HT4 receptor agonist[1] |
| Main uses | Long-term constipation[1] |
| Side effects | Headache, nausea, diarrhea, abdominal pain[1] |
| Pregnancy category |
|
| Routes of use | By mouth |
| Typical dose | 2 mg[1] |
| External links | |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a619011 |
| Legal | |
| License data |
|
| Legal status | |
| Chemical and physical data | |
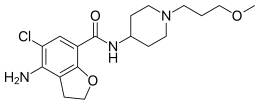
| Formula | C18H26ClN3O3 |
| Molar mass | 367.87 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Prucalopride, sold under the brand name Resolor among others, is a medication used to treat chronic constipation of unknown cause.[1][2] It may be used when other laxatives are not effective; though is not recommended in Scotland.[3] It is taken by mouth.[1]
Common side effects include headache, nausea, diarrhea, and abdominal pain.[1] Other side effects may include allergic reactions and suicide.[2] Safety in pregnancy is unclear.[2] It is a 5-HT4 receptor agonist which works by stimulating movement of the gut.[1]
Prucalopride was approved for medical use in Europe in 2009,[1] Canada in 2011,[4] Israel in 2014,[5] and the United States in 2018.[2] In the United Kingdom four weeks of medication costs the NHS about £60 as of 2021.[3] In the United States this amount costs about 430 USD.[6]
Medical uses
The primary measure of efficacy in the clinical trials is three or more spontaneous complete bowel movements per week; a secondary measure is an increase of at least one complete spontaneous bowel movement per week.[7][8][9] Further measures are improvements in PAC-QOL[10] (a quality of life measure) and PAC-SYM[11] (a range of stool, abdominal, and rectal symptoms associated with chronic constipation). Infrequent bowel movements, bloating, straining, abdominal pain, and defecation urge with inability to evacuate can be severe symptoms, significantly affecting quality of life.[12][13][14][15][16]
In three large clinical trials, 12 weeks of treatment with prucalopride 2 and 4 mg/day resulted in a higher proportion of patients reaching the primary efficacy endpoint of an average of ≥3 spontaneous complete bowel movements than with placebo.[7][8][9] There was also significantly improved bowel habit and associated symptoms, patient satisfaction with bowel habit and treatment, and HR-QOL in patients with severe chronic constipation, including those who did not experience adequate relief with prior therapies (>80% of the trial participants).[7][8][9] The improvement in patient satisfaction with bowel habit and treatment was maintained during treatment for up to 24 months; prucalopride therapy was generally well tolerated.[17][18]
Dosage
It is typically taken at a dose of 2 mg once per day.[1] People over 65 may start at 1 mg per day.[1]
Contraindications
Prucalopride is contraindicated where there is hypersensitivity to the active substance or to any of the excipients, renal impairment requiring dialysis, intestinal perforation or obstruction due to structural or functional disorder of the gut wall, obstructive ileus, severe inflammatory conditions of the intestinal tract, such as Crohn's disease, and ulcerative colitis and toxic megacolon/megarectum.[19]
Side effects
Prucalopride has been given orally to ~2700 patients with chronic constipation in controlled clinical trials. The most frequently reported side effects are headache and gastrointestinal symptoms (abdominal pain, nausea or diarrhea). Such reactions occur predominantly at the start of therapy and usually disappear within a few days with continued treatment.[19]
Mechanism of action
Prucalopride, a first in class dihydro-benzofuran-carboxamide, is a selective, high affinity serotonin (5-HT4) receptor agonist with enterokinetic activities.[19] Prucalopride alters colonic motility patterns via serotonin 5-HT4 receptor stimulation: it stimulates colonic mass movements, which provide the main propulsive force for defecation.
The observed effects are exerted via highly selective action on 5-HT4 receptors:[19] prucalopride has >150-fold higher affinity for 5-HT4 receptors than for other receptors.[20][21] Prucalopride differs from other 5-HT4 agonists such as tegaserod and cisapride, which at therapeutic concentrations also interact with other receptors (5-HT1B/D and the cardiac human ether-a-go-go K+ or hERG channel respectively) and this may account for the adverse cardiovascular events that have resulted in the restricted availability of these drugs.[21] Clinical trials evaluating the effect of prucalopride on QT interval and related adverse events have not demonstrated significant differences compared with placebo.[19]
Pharmacokinetics
Prucalopride is rapidly absorbed (Cmax attained 2–3 hours after single 2 mg oral dose) and is extensively distributed. Metabolism is not the major route of elimination. In vitro, human liver metabolism is very slow and only minor amounts of metabolites are found. A large fraction of the active substance is excreted unchanged (about 60% of the administered dose in urine and at least 6% in feces). Renal excretion of unchanged prucalopride involves both passive filtration and active secretion. Plasma clearance averages 317 ml/min, terminal half-life is 24–30 hours,[22] and steady-state is reached within 3–4 days. On once daily treatment with 2 mg prucalopride, steady-state plasma concentrations fluctuate between trough and peak values of 2.5 and 7 ng/ml, respectively.[19]
In vitro data indicate that prucalopride has a low interaction potential, and therapeutic concentrations of prucalopride are not expected to affect the CYP-mediated metabolism of co-medicated medicinal products.[19]
Society and culture
Approval
In the European Economic Area, prucalopride was originally approved for the symptomatic treatment of chronic constipation in women in whom laxatives fail to provide adequate relief.[19] Subsequently, it has been approved by the European Commission for use in adults – that is, including male patients – for the same indication.[23]
Research
Small clinical trials suggested that prucalopride administration results in the 5-HT4 receptor agonism-associated memory enhancing in healthy participants improving their ability to recall and increasing neural activation in the hippocampus and functionally related areas.[24][25]
References
- ↑ 1.00 1.01 1.02 1.03 1.04 1.05 1.06 1.07 1.08 1.09 1.10 "Resolor". Archived from the original on 31 January 2010. Retrieved 29 October 2021.
- ↑ 2.0 2.1 2.2 2.3 "Prucalopride Monograph for Professionals". Drugs.com. Archived from the original on 28 January 2021. Retrieved 29 October 2021.
- ↑ 3.0 3.1 BNF 81: March-September 2021. BMJ Group and the Pharmaceutical Press. 2021. p. 64. ISBN 978-0857114105.
- ↑ "Health Canada, Notice of Decision for Resotran". hc-sc.gc.ca. Archived from the original on 18 March 2017. Retrieved 1 May 2018.
- ↑ "Digestive Remedies in Israel". www.euromonitor.com. Archived from the original on 13 March 2018. Retrieved 1 May 2018.
- ↑ "Motegrity Prices, Coupons & Savings Tips - GoodRx". GoodRx. Retrieved 29 October 2021.
- ↑ 7.0 7.1 7.2 Tack J, van Outryve M, Beyens G, Kerstens R, Vandeplassche L (March 2009). "Prucalopride (Resolor) in the treatment of severe chronic constipation in patients dissatisfied with laxatives". Gut. 58 (3): 357–65. doi:10.1136/gut.2008.162404. PMID 18987031. S2CID 206948212.
- ↑ 8.0 8.1 8.2 Camilleri M, Kerstens R, Rykx A, Vandeplassche L (May 2008). "A placebo-controlled trial of prucalopride for severe chronic constipation". The New England Journal of Medicine. 358 (22): 2344–54. doi:10.1056/NEJMoa0800670. PMID 18509121.
- ↑ 9.0 9.1 9.2 Quigley EM, Vandeplassche L, Kerstens R, Ausma J (February 2009). "Clinical trial: the efficacy, impact on quality of life, and safety and tolerability of prucalopride in severe chronic constipation--a 12-week, randomized, double-blind, placebo-controlled study". Alimentary Pharmacology & Therapeutics. 29 (3): 315–28. doi:10.1111/j.1365-2036.2008.03884.x. PMID 19035970. S2CID 40122406.
- ↑ Marquis P, De La Loge C, Dubois D, McDermott A, Chassany O (May 2005). "Development and validation of the Patient Assessment of Constipation Quality of Life questionnaire". Scandinavian Journal of Gastroenterology. 40 (5): 540–51. doi:10.1080/00365520510012208. PMID 16036506. S2CID 34620643.
- ↑ Frank L, Kleinman L, Farup C, Taylor L, Miner P (September 1999). "Psychometric validation of a constipation symptom assessment questionnaire". Scandinavian Journal of Gastroenterology. 34 (9): 870–7. doi:10.1080/003655299750025327. PMID 10522604.
- ↑ Johanson JF, Kralstein J (March 2007). "Chronic constipation: a survey of the patient perspective". Alimentary Pharmacology & Therapeutics. 25 (5): 599–608. doi:10.1111/j.1365-2036.2006.03238.x. PMID 17305761. S2CID 25400560.
- ↑ Koch A, Voderholzer WA, Klauser AG, Müller-Lissner S (August 1997). "Symptoms in chronic constipation". Diseases of the Colon and Rectum. 40 (8): 902–6. doi:10.1007/BF02051196. PMID 9269805. S2CID 28066729.
- ↑ McCrea GL, Miaskowski C, Stotts NA, Macera L, Paul SM, Varma MG (April 2009). "Gender differences in self-reported constipation characteristics, symptoms, and bowel and dietary habits among patients attending a specialty clinic for constipation". Gender Medicine. 6 (1): 259–71. doi:10.1016/j.genm.2009.04.007. PMID 19467522.
- ↑ Pare P, Ferrazzi S, Thompson WG, Irvine EJ, Rance L (November 2001). "An epidemiological survey of constipation in canada: definitions, rates, demographics, and predictors of health care seeking". The American Journal of Gastroenterology. 96 (11): 3130–7. PMID 11721760.
- ↑ Wald A, Scarpignato C, Kamm MA, Mueller-Lissner S, Helfrich I, Schuijt C, et al. (July 2007). "The burden of constipation on quality of life: results of a multinational survey". Alimentary Pharmacology & Therapeutics. 26 (2): 227–36. doi:10.1111/j.1365-2036.2007.03376.x. PMID 17593068. S2CID 19457828.
- ↑ Camilleri M, Beyens G, Kerstens R, Vandeplassche L (2009). "Long-term follow-up of safety and satisfaction with bowel function in response to oral prucalopride in patients with chronic constipation [Abstract]". Gastroenterology. 136 (Suppl 1): 160. doi:10.1016/s0016-5085(09)60143-8.
- ↑ Van Outryve MJ, Beyens G, Kerstens R, Vandeplassche L (2008). "Long-term follow-up study of oral prucalopride (Resolor) administered to patients with chronic constipation [Abstract T1400]". Gastroenterology. 134 (4 (suppl 1)): A547. doi:10.1016/s0016-5085(08)62554-8.
- ↑ 19.0 19.1 19.2 19.3 19.4 19.5 19.6 19.7 SmPC. Summary of product characteristics Resolor (prucalopride) October, 2009: 1-9.
- ↑ Briejer MR, Bosmans JP, Van Daele P, Jurzak M, Heylen L, Leysen JE, et al. (June 2001). "The in vitro pharmacological profile of prucalopride, a novel enterokinetic compound". European Journal of Pharmacology. 423 (1): 71–83. doi:10.1016/S0014-2999(01)01087-1. PMID 11438309.
- ↑ 21.0 21.1 De Maeyer JH, Lefebvre RA, Schuurkes JA (February 2008). "5-HT4 receptor agonists: similar but not the same". Neurogastroenterology and Motility. 20 (2): 99–112. doi:10.1111/j.1365-2982.2007.01059.x. PMID 18199093. S2CID 43095011.
- ↑ Frampton JE (2009). "Prucalopride". Drugs. 69 (17): 2463–76. doi:10.2165/11204000-000000000-00000. PMID 19911858.
- ↑ "Shire Receives European Approval to Use Resolor® (prucalopride) in Men for the Symptomatic Treatment of Chronic Constipation". www.shire.com. Archived from the original on 21 November 2017. Retrieved 1 May 2018.
- ↑ Murphy, S. E.; Wright, L. C.; Browning, M.; Cowen, P. J.; Harmer, C. J. (2020). "A role for 5-HT4 receptors in human learning and memory". Psychological Medicine. 50 (16): 2722–2730. doi:10.1017/S0033291719002836. ISSN 0033-2917. Archived from the original on 2021-10-08. Retrieved 2021-10-14.
- ↑ de Cates, A. N.; Wright, L. C.; Martens, M. A. G.; Gibson, D.; Türkmen, C.; Filippini, N.; Cowen, P. J.; Harmer, C. J.; Murphy, S. E. (2021). "Déjà-vu? Neural and behavioural effects of the 5-HT4 receptor agonist, prucalopride, in a hippocampal-dependent memory task". Translational Psychiatry. 11 (1): 1–9. doi:10.1038/s41398-021-01568-4. ISSN 2158-3188. Archived from the original on 2021-10-15. Retrieved 2021-10-14.
External links
| External sites: | |
|---|---|
| Identifiers: |
|
- "Prucalopride succinate". Drug Information Portal. U.S. National Library of Medicine. Archived from the original on 2020-10-21. Retrieved 2021-10-14.
- Pages using duplicate arguments in template calls
- Drugs with non-standard legal status
- Articles with changed InChI identifier
- Chemical articles with unknown parameter in Infobox drug
- Chemical articles without CAS registry number
- Articles without EBI source
- Chemical pages without ChemSpiderID
- Chemical pages without DrugBank identifier
- Articles without KEGG source
- Articles without UNII source
- Chemicals using indexlabels
- Drugs missing an ATC code
- Drug has EMA link
- Drugboxes which contain changes to verified fields
- Drugboxes which contain changes to watched fields
- Articles with changed ChemSpider identifier
- Articles with changed KEGG identifier
- Motility stimulants
- Serotonin receptor agonists
- Benzofurans
- Piperidines
- 4-Amino-N-(3-(diethylamino)propyl)-2-methoxybenzamides
- Chloroarenes
- RTT