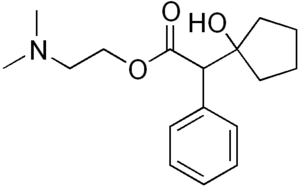
Cyclopentolate
 | |
| Names | |
|---|---|
| Other names | Cyclopentolate hydrochloride |
| |
| Clinical data | |
| Drug class | Muscarinic antagonist[1] |
| Main uses | Eye examination, uveitis (iritis)[2][1] |
| Side effects | Increased eye pressure, eye irritation, blurry vision, sensitivity to light[1] |
| Pregnancy category |
|
| Routes of use | Eye drop |
| External links | |
| AHFS/Drugs.com | Monograph |
| Chemical and physical data | |
| Formula | C17H25NO3 |
| Molar mass | 291.391 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Cyclopentolate is a medication used to dilate the eyes to help with eye examination.[1] It may also be used to help with the pain of uveitis (iritis).[2] It is applied as an eye drop.[2] Maximal effect occurs within an hour and usually lasts about a day.[1]
Common side effects include increased eye pressure, eye irritation, blurry vision, and sensitivity to light.[1] Other side effects may include behavioral changes and allergic reactions.[1] Safety in pregnancy is unclear.[1] It is a muscarinic antagonist.[1]
Cyclopentolate was approved for medical use in the United States in 1974.[1] It is available as a generic medication.[3] It is on the World Health Organization's List of Essential Medicines as an alternative to tropicamide.[4] In the United Kingdom 10 ml (20 doses) costs the NHS about £11 as of 2021.[2] This amount in the United States is about 18 USD.[3]
Medical uses

After instillation of cyclopentolate, pupil dilation (mydriasis) typically lasts up to 24 hours, while paralysis of the ciliary muscle (cycloplegia) typically lasts 6-24 hours.[5] During this time, patients may be more light sensitive than normal and may notice close objects blurred (and possibly distant objects blurred, depending on the patient's visual system). Cyclopentolate is often chosen as a milder, shorter-lasting, cycloplegic alternative to atropine, another cycloplegic agent which lasts much longer. Tropicamide is an even shorter-lasting cycloplegic than cyclopentolate, but is less reliable for finding latent hyperopia. Cyclopentolate drops act rapidly to dilate the pupil.[6]
Cyclopentolate or atropine can also be administered to reverse muscarinic and central nervous system effects of indirect cholinomimetic (anti-AChase) administration.
Side effects
The side effects of cyclopentolate are similar to the side and adverse effects of other anticholinergic medications. Because of that, extra caution should be taken when prescribing cyclopentolate to patients who are already taking other anticholinergic drugs. A possible ocular (eye-related) side effect is increase in pressure inside the eye, which is of particular concern when there is a predisposition toward or a presence of glaucoma. Other ocular side effects can include burning sensations, discomfort with bright light (photophobia), blurred vision, irritation, inflammation of the eye mucous membranes (conjunctivitis), inflammation of the cornea of the eye (keratitis), and other issues. Nonocular (not eye-related) side and adverse effects can include neuropsychiatric symptoms[7] like subtle concentration and memory problems, subtle decision-making problems, drowsiness, and more pronounced disorientation to time and place, confusion, disturbances of speech and movement, hyperactivity, restlessness, and seizures. Temporary psychosis[8] can develop that includes hallucinations, particularly when higher doses are used in children or older adults[9] on other anticholinergic medications.[10] Patients with dementia of the Alzheimer's type can experience worsening of their dementia symptoms. Additional side and adverse effects can include skin flushing, skin rashes, gastrointestinal problems, increased heart beat (tachycardia), increased body temperature (hyperpyrexia), blood vessel dilation, urinary retention, dry mouth and reduced sweating, and reduced bronchial secretions. Severe poisoning with cyclopentolate may result in coma, paralysis of breathing, and death. Cyclopentolate derivatives can be used as an antidote for organophosphate poisoning.[11] [12] [13] [14] [15]
Lethality of cyclopentolate has been studied in rodents. The LD50 (the dose at which 50% of animals die from the drug) is approximately 4000 mg/kg in rats and 960 mg/kg in mice. Readily recognizable symptoms of overdose include tachycardia, dizziness, dry mouth, behavioral disturbances, poor coordination, and drowsiness.
Cycloplegia is necessary in cases of suspected latent hyperopia (or "over-focusing") so that an ophthalmologist or optometrist can accurately measure how much a person has to flex their focusing muscle (accommodation) in order to see in the distance and up-close. Correction of latent hyperopia in children can often prevent, or sometimes correct, unwanted eye turns (strabismus), some forms of refractive amblyopia, and may alleviate eye strain or frontal headaches caused by prolonged near-work. Cycloplegia is also helpful in relieving accommodative spasm.
History
Cyclopentolate was first synthesized in 1952 as a chemical analogue of atropine. It was one of several derivatives of an analogue to tropic acid which were tested for pharmacological action "in a search for new and better antispasmodic agents."[16]
Society and culture
Brand names
Brand names for cyclopentolate include Cyclogyl, Cylate, Mydrilate, and Pentolair.[17]
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 1.6 1.7 1.8 1.9 "Cyclopentolate Monograph for Professionals". Drugs.com. Archived from the original on 29 September 2021. Retrieved 7 January 2022.
- ↑ 2.0 2.1 2.2 2.3 BNF 81: March-September 2021. BMJ Group and the Pharmaceutical Press. 2021. p. 1222. ISBN 978-0857114105.
- ↑ 3.0 3.1 "Cyclopentolate Prices, Coupons & Savings Tips - GoodRx". GoodRx. Retrieved 7 January 2022.
- ↑ World Health Organization (2021). World Health Organization model list of essential medicines: 22nd list (2021). Geneva: World Health Organization. hdl:10665/345533. WHO/MHP/HPS/EML/2021.02.
- ↑ Clinical Ocular Pharmacology, 5th edition, Bartlett, 2008. Chapter 9: Cycloplegics. Table 9-1: Mydriatic and Cycloplegic Properties of Anticholinergic Agents. p.127.
- ↑ "Cyclogyl Eye Drops Medsafe data sheet New Zealand" (PDF). 11 January 2017. Archived (PDF) from the original on 9 April 2017. Retrieved 3 June 2017.
- ↑ Derinoz, O., & Er, A. (2012). Inability to walk, disequilibrium, incoherent speech, disorientation following the instillation of 1% cyclopentaolate eyedrops: Case report. Pediatric Emergency Care, 28(1), 59-60.
- ↑ Rajappa, N., Patra, S., Bhalsing, S., & Lune, A. A. (2014). A case of acute psychosis induced by topical cyclopentolate eye drops in an elderly patient. Medical journal of Dr. D. Y. Patil Vidyapeeth, 7(1), 68-69.
- ↑ Barker, D. B., & Solomon, D. A. (1990). The potential for mental status changes associated with systemic absorption of anticholinergic ophthalmic medication: Concerns in the elderly. Annals of Pharmacotherapy, 24(9), 847-850.
- ↑ Carpenter, W. T. (1967). Precipitous mental deterioration following cycloplegia with 0.2% cyclopentolate HCl. Archives of Ophthalmology, 78(4), 445-447.
- ↑ Bryant, S.M., Rhee, J. W., Thompson, T. M., et al. (2009). Paenteral ophthalmic topicamide or cyclopentolate protects rats from lethal organophosphate poisoning. American Journal of Therapeutics, 16(3), 231-234.
- ↑ Nyhan, W. L., et al. (1973). Systemic cyclopentolate (Cyclogyl) toxicity in the newborn infant. The Journal of Pediatrics, 82(3), 501-505.
- ↑ Fitzgerald, D.A., et al. (1990). Seizures associated with 1% cyclopentolate eyedrops. Journal of Pediatrics and Child Health, 26(2), https://doi.org/10.1111/j.1440-1754.1990.tb02399.x
- ↑ Bhatia, S.S., Vidyashankar, C., Sharma, R. K., & Dubey, A. K. (2000). Systemic toxicity with cyclopentolate eye drops. Indian Pediatrics, 37, 329-331.
- ↑ Ozgun, U., et al. (2014). Fatal necrotising enterocolitis due to mydriatic eye drops. Journal of the College of Physicians and Surgeons Pakistan, 24(SS2), S147-S149.
- ↑ Treves GR, Testa FC (1952). "Basic Esters and Quaternary Derivatives of β-Hydroxy Acids as Antispasmodics1". Journal of the American Chemical Society. 74 (1): 46–48. doi:10.1021/ja01121a012. ISSN 0002-7863.
- ↑ "cyclopentolate hydrochloride solution - ophthalmic, Cyclogyl, Cylate, Pentolair". Archived from the original on December 12, 2018. Retrieved June 15, 2012.
External links
| Identifiers: |
|
|---|
- Pages using duplicate arguments in template calls
- Chemical articles with unknown parameter in Infobox drug
- Chemical articles without CAS registry number
- Articles without EBI source
- Chemical pages without ChemSpiderID
- Chemical pages without DrugBank identifier
- Articles without KEGG source
- Articles without UNII source
- Drugs missing an ATC code
- Drugs with no legal status
- Articles containing unverified chemical infoboxes
- Articles with changed EBI identifier
- World Health Organization essential medicines (alternatives)
- Ophthalmology drugs
- Dimethylamino compounds
- Acetate esters
- Muscarinic antagonists
- Tertiary alcohols
- Cyclopentanes
- RTT