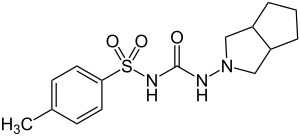
Gliclazide
 | |
 | |
| Names | |
|---|---|
| Trade names | Diamicron, Diaprel, Azukon, others[1] |
| |
| Clinical data | |
| Main uses | Type 2 diabetes[2] |
| Pregnancy category |
|
| Defined daily dose | 60 mg[3] |
| External links | |
| AHFS/Drugs.com | Micromedex Detailed Consumer Information |
| Legal | |
| Legal status |
|
| Pharmacokinetics | |
| Elimination half-life | 10.4 hours |
| Chemical and physical data | |
| Formula | C15H21N3O3S |
| Molar mass | 323.41 g·mol−1 |
| 3D model (JSmol) | |
| Melting point | 180 to 182 °C (356 to 360 °F) |
| |
| |
Gliclazide, sold under the brand name Diamicron among others, is an anti-diabetic medication used to treat diabetes mellitus type 2.[4] It is used when dietary changes, exercise, and weight loss are not enough.[5] It is taken by mouth.[4]
Side effect may include low blood sugar, vomiting, abdominal pain, rash, and liver problems.[5][4] Use by those with significant kidney problems or liver problems or who are pregnant is not recommended.[4][5] Gliclazide is in the sulfonylurea family of medications.[4] It works mostly by increasing the release of insulin.[4]
Gliclazide was patented in 1966 and approved for medical use in 1972.[6] It is on the World Health Organization's List of Essential Medicines.[7] The wholesale cost in the developing world is about US$2.46–3.92 per month.[8] In the United Kingdom a month of medication costs the NHS about £2.12.[4] It is not available for sale in the United States.[9]
Medical uses
Gliclazide is used for control of hyperglycemia in gliclazide-responsive diabetes mellitus of stable, mild, non-ketosis prone, type 2 diabetes. It is used when diabetes cannot be controlled by proper dietary management and exercise or when insulin therapy is not appropriate.[citation needed] National Kidney Foundation (2012 Update) claims that Gliclazide does not require dosage uptitration even in end stage kidney disease.
Dosage
The defined daily dose is 60 mg.[3] It is generally started at 40 mg once per day for the initial two weeks and than increased by 40 mg at two week intervals to a typical dose of 80 to 160 mg.[2]
Contraindications
- Type 1 diabetes[10]
- Hypersensitivity to sulfonylureas[10]
- Severe kidney or liver failure[10] (But relatively useful in mild kidney impairment e.g. CKD stage 3)
- Pregnancy and lactation[10]
Adverse effects
- Hypoglycemia - while it was shown to have the same efficacy as glimepiride, one of the newer sulfonylureas, the European GUIDE study has shown that it has approximately 50% less hypoglycaemic confirmed episodes in comparison with glimepiride.[11]
Interactions
Hyperglycemic action may be caused by danazol, chlorpromazine, glucocorticoids, progestogens, or β-2 agonists. Its hypoglycemic action may be potentiated by phenylbutazone, alcohol, fluconazole, β-blockers, and possibly ACE inhibitors. It has been found that rifampin increases gliclazide metabolism in humans in vivo.[12]
Overdose
Gliclazide overdose may cause severe hypoglycemia, requiring urgent administration of glucose by IV and monitoring.[citation needed]
Mechanism of action
Gliclazide selectively binds to sulfonylurea receptors (SUR-1) on the surface of the pancreatic beta-cells. It was shown to provide cardiovascular protection as it does not bind to sulfonylurea receptors (SUR-2A) in the heart.[13] This binding effectively closes these K+ ion channels. This decreases the efflux of potassium from the cell which leads to the depolarization of the cell. This causes voltage dependent Ca2+ ion channels to open increasing the Ca2+ influx. The calcium can then bind to and activate calmodulin which in turn leads to exocytosis of insulin vesicles leading to insulin release.[citation needed] The mouse model of MODY diabetes suggested that the reduced gliclazide clearance stands behind their therapeutic success in human MODY patients, but Urbanova et al. found that human MODY patients respond differently and that there was no consistent decrease in gliclazide clearance in randomly selected HNF1A-MODY and HNF4A-MODY patients.[14]
Its classification has been ambiguous, as literature uses it as both a first-generation[15] and second-generation[16] sulfonylurea.
Properties
According to the Biopharmaceutical Classification System (BCS), gliclazide falls under the BCS Class II drug, which is poorly soluble and highly permeable.
Water solubility = 0.027 mg/L[17]
- Hypoglycemic sulfonylurea, restoring first peak of insulin secretion, increasing insulin sensitivity.[citation needed]
- Glycemia-independent hemovascular effects, antioxidant effect.[citation needed]
- No active circulating metabolites.[citation needed]
Metabolism
Gliclazide undergoes extensive metabolism to several inactive metabolites in human beings, mainly methylhydroxygliclazide and carboxygliclazide. CYP2C9 is involved in the formation of hydroxygliclazide in human liver microsomes and in a panel of recombinant human P450s in vitro.[18][19] But the pharmacokinetics of gliclazide MR are affected mainly by CYP2C19 genetic polymorphism instead of CYP2C9 genetic polymorphism.[20][21]
References
- ↑ "Gliclazide - Drugs.com". www.drugs.com. Archived from the original on 27 December 2016. Retrieved 27 December 2016.
- ↑ 2.0 2.1 "GLICLAZIDE oral - Essential drugs". medicalguidelines.msf.org. Archived from the original on 28 August 2021. Retrieved 30 August 2020.
- ↑ 3.0 3.1 "WHOCC - ATC/DDD Index". www.whocc.no. Archived from the original on 22 January 2021. Retrieved 30 August 2020.
- ↑ 4.0 4.1 4.2 4.3 4.4 4.5 4.6 British National Formulary : BNF 69 (69 ed.). British Medical Association. 2015. p. 474. ISBN 9780857111562.
- ↑ 5.0 5.1 5.2 "Bilxona 30mg Modified-release Tablets - Summary of Product Characteristics (SPC) - (eMC)". www.medicines.org.uk. 29 August 2016. Archived from the original on 27 December 2016. Retrieved 27 December 2016.
- ↑ Fischer, Janos; Ganellin, C. Robin (2006). Analogue-based Drug Discovery. John Wiley & Sons. p. 449. ISBN 9783527607495. Archived from the original on 2016-12-27.
- ↑ World Health Organization (2019). World Health Organization model list of essential medicines: 21st list 2019. Geneva: World Health Organization. hdl:10665/325771. WHO/MVP/EMP/IAU/2019.06. License: CC BY-NC-SA 3.0 IGO.
- ↑ "Gliclazide". International Drug Price Indicator Guide. Archived from the original on 22 January 2018. Retrieved 8 December 2016.
- ↑ "Gliclazide Advanced Patient Information - Drugs.com". www.drugs.com. Archived from the original on 27 December 2016. Retrieved 27 December 2016.
- ↑ 10.0 10.1 10.2 10.3 "GLICLAZIDE 60 MG MR TABLETS DRUG LEAFLET". Drugs.com. Archived from the original on 21 April 2019. Retrieved 23 March 2020.
- ↑ Schernthaner, G.; Grimaldi, A.; Di Mario, U.; Drzewoski, J.; Kempler, P.; Kvapil, M.; Novials, A.; Rottiers, R.; et al. (2004). "GUIDE study: Double-blind comparison of once-daily gliclazide MR and glimepiride in type 2 diabetic patients". European Journal of Clinical Investigation. 34 (8): 535–42. doi:10.1111/j.1365-2362.2004.01381.x. hdl:1874/10657. PMID 15305887.
- ↑ Park, J; Kim, KA; Park, PW; Park, CW; Shin, JG (2003). "Effect of rifampin on the pharmacokinetics and pharmacodynamics of gliclazide". Clinical Pharmacology & Therapeutics. 74 (4): 334–40. doi:10.1016/S0009-9236(03)00221-2. PMID 14534520.
- ↑ Lawrence, C. L.; Proks, P.; Rodrigo, G. C.; Jones, P.; Hayabuchi, Y.; Standen, N. B.; Ashcroft, F. M. (2001). "Gliclazide produces high-affinity block of KATP channels in mouse isolated pancreatic beta cells but not rat heart or arterial smooth muscle cells". Diabetologia. 44 (8): 1019–25. doi:10.1007/s001250100595. PMID 11484080.
- ↑ Urbanova, J.; et al. (2015). "Half-Life of Sulfonylureas in HNF1A and HNF4A Human MODY Patients is not Prolonged as Suggested by the Mouse Hnf1a-/- Model". Current Pharmaceutical Design. 21 (39): 5736–5748. doi:10.2174/1381612821666151008124036. PMID 26446475.
- ↑ Ballagi-Pordány, György; Köszeghy, Anna; Koltai, Mária-Zsófia; Aranyi, Zoltán; Pogátsa, Gábor (1990). "Divergent cardiac effects of the first and second generation hypoglycemic sulfonylurea compounds". Diabetes Research and Clinical Practice. 8 (2): 109–14. doi:10.1016/0168-8227(90)90020-T. PMID 2106423.
- ↑ Shimoyama, Tatsuhiro; Yamaguchi, Shinya; Takahashi, Kazuto; Katsuta, Hidenori; Ito, Eisuke; Seki, Hiroyuki; Ushikawa, Kenji; Katahira, Hiroshi; et al. (2006). "Gliclazide protects 3T3L1 adipocytes against insulin resistance induced by hydrogen peroxide with restoration of GLUT4 translocation". Metabolism. 55 (6): 722–30. doi:10.1016/j.metabol.2006.01.019. PMID 16713429.
- ↑ Gopal Venkatesh Shavi et al. Enhanced dissolution and bioavailability of gliclazide using solid dispersion techniques; International Journal of Drug Delivery 2 (2010) 49-57
- ↑ Rieutord, A; Stupans, I; Shenfield, GM; Gross, AS (1995). "Gliclazide hydroxylation by rat liver microsomes". Xenobiotica. 25 (12): 1345–54. doi:10.3109/00498259509061922. PMID 8719909.
- ↑ Elliot, David J.; Lewis, Benjamin C.; Gillam, Elizabeth M. J.; Birkett, Donald J.; Gross, Annette S.; Miners, John O.; Miners, JO (2007). "Identification of the human cytochromes P450 catalysing the rate-limiting pathways of gliclazide elimination". British Journal of Clinical Pharmacology. 64 (4): 450–7. doi:10.1111/j.1365-2125.2007.02943.x. PMC 2048545. PMID 17517049.
- ↑ Zhang, Yifan; Si, Dayong; Chen, Xiaoyan; Lin, Nan; Guo, Yingjie; Zhou, Hui; Zhong, Dafang (2007). "Influence of CYP2C9 and CYP2C19 genetic polymorphisms on pharmacokinetics of gliclazide MR in Chinese subjects". British Journal of Clinical Pharmacology. 64 (1): 67–74. doi:10.1111/j.1365-2125.2007.02846.x. PMC 2000619. PMID 17298483.
- ↑ Xu, H; Williams, K M; Liauw, W S; Murray, M; Day, R O; McLachlan, A J (2009). "Effects of St John's wort and CYP2C9 genotype on the pharmacokinetics and pharmacodynamics of gliclazide". British Journal of Pharmacology. 153 (7): 1579–86. doi:10.1038/sj.bjp.0707685. PMC 2437900. PMID 18204476.
External links
| External sites: | |
|---|---|
| Identifiers: |
|
- Pages using duplicate arguments in template calls
- Chemical articles with unknown parameter in Infobox drug
- Chemical articles without CAS registry number
- Articles without EBI source
- Chemical pages without ChemSpiderID
- Chemical pages without DrugBank identifier
- Articles without KEGG source
- Articles without UNII source
- Drugs missing an ATC code
- Drugboxes which contain changes to watched fields
- All articles with unsourced statements
- Articles with unsourced statements from September 2011
- Articles with invalid date parameter in template
- Potassium channel blockers
- Benzenesulfonylureas
- Laboratoires Servier
- World Health Organization essential medicines
- RTT
- Cyclopentanes
- P-Tosyl compounds