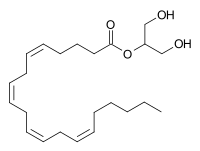
2-Arachidonoylglycerol
| |
| |
| Names | |
|---|---|
| IUPAC name
2-O-[(5Z,8Z,11Z,14Z)-Icosa-5,8,11,14-tetraenoyl]glycerol
| |
| Systematic IUPAC name
1,3-Dihydroxypropan-2-yl (5Z,8Z,11Z,14Z)-icosa-5,8,11,14-tetraenoate | |
| Other names
2-AG, 2-arachidonoylglycerol
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C23H38O4 | |
| Molar mass | 378.3 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
2-Arachidonoylglycerol (2-AG) is an endocannabinoid, an endogenous agonist of the CB1 receptor and the primary endogenous ligand for the CB2 receptor.[1][2] It is an ester formed from the omega-6 fatty acid arachidonic acid and glycerol. It is present at relatively high levels in the central nervous system, with cannabinoid neuromodulatory effects. It has been found in maternal bovine and human milk.[3] The chemical was first described in 1994–1995, although it had been discovered some time before that. The activities of phospholipase C (PLC) and diacylglycerol lipase (DAGL) mediate its formation.[4] 2-AG is synthesized from arachidonic acid-containing diacylglycerol (DAG).
Occurrence
2-AG, unlike anandamide (another endocannabinoid), is present at relatively high levels in the central nervous system; it is the most abundant molecular species of monoacylglycerol found in mouse and rat brain (~5–10 nmol/g tissue).[2][5] Detection of 2-AG in brain tissue is complicated by the relative ease of its isomerization to 1-AG during standard lipid extraction conditions. It has been found in maternal bovine as well as human milk.[6][7][8]
Discovery
2-AG was discovered by Raphael Mechoulam and his student Shimon Ben-Shabat.[9] 2-AG was a known chemical compound but its occurrence in mammals and its affinity for the cannabinoid receptors were first described in 1994–1995. A research group at Teikyo University reported the affinity of 2-AG for the cannabinoid receptors in 1994–1995,[10][11] but the isolation of 2-AG in the canine gut was first reported in 1995 by the research group of Raphael Mechoulam at the Hebrew University of Jerusalem, which additionally characterized its pharmacological properties in vivo.[12] 2-Arachidonoylglycerol, next with Anandamide, was the second endocannabinoid discovered. The cannabinoid established the existence of a cannabinoid neuromodulatory system in the nervous system.[13]
Pharmacology
Unlike anandamide, formation of 2-AG is calcium-dependent and is mediated by the activities of phospholipase C (PLC) and diacylglycerol lipase (DAGL).[2] 2-AG acts as a full agonist at the CB1 receptor.[14] At a concentration of 0.3 nM, 2-AG induces a rapid, transient increase in intracellular free calcium in NG108-15 neuroblastoma X glioma cells through a CB1 receptor-dependent mechanism.[2] 2-AG is hydrolyzed in vitro by monoacylglycerol lipase (MAGL), fatty acid amide hydrolase (FAAH), and the uncharacterized serine hydrolase enzymes ABHD2,[15] ABHD6 and ABHD12.[16] The exact contribution of each of these enzymes to the termination of 2-AG signaling in vivo is unknown, though it is estimated that MAGL is responsible for ~85% of this activity in the brain.[17] There have been identified transport proteins for 2-arachidonoylglycerol and anandamide. These include the heat shock proteins (Hsp70s) and fatty acid binding proteins (FABPs).[18][19]
Biosynthesis
2-Arachidonoylglycerol is synthesized from arachidonic acid-containing diacylglycerol (DAG), which is derived from the increase of inositol phospholipid metabolism by the action of diacylglycerol lipase. The molecule can also be formed from pathways like the hydrolysis derived (by diglyceride) from both phosphatidylcholine (PC) and phosphatidic acid (PAs) by the action of DAG lipase and the hydrolysis of arachidonic acid-containing lysophosphatidic acid by the action of a phosphatase.[20]
See also
References
Notes
- ^ Stella N, Schweitzer P, Piomelli D (August 1997). "A second endogenous cannabinoid that modulates long-term potentiation" (PDF). Nature. 388 (6644): 773–8. Bibcode:1997Natur.388..773S. doi:10.1038/42015. PMID 9285589. S2CID 4422311.
- ^ a b c d Sugiura T, Kodaka T, Nakane S, et al. (January 1999). "Evidence that the cannabinoid CB1 receptor is a 2-arachidonoylglycerol receptor. Structure-activity relationship of 2-arachidonoylglycerol, ether-linked analogues, and related compounds". The Journal of Biological Chemistry. 274 (5): 2794–801. doi:10.1074/jbc.274.5.2794. PMID 9915812.
- ^ Berrendero, F.; Sepe, N.; Ramos, J. A.; Di Marzo, V.; Fernández-Ruiz, J. J. (1999-09-01). "Analysis of cannabinoid receptor binding and mRNA expression and endogenous cannabinoid contents in the developing rat brain during late gestation and early postnatal period". Synapse (New York, N.Y.). 33 (3): 181–191. doi:10.1002/(SICI)1098-2396(19990901)33:3<181::AID-SYN3>3.0.CO;2-R. ISSN 0887-4476. PMID 10420166. S2CID 39220005.
- ^ Witting, Anke; Walter, Lisa; Wacker, Jennifer; Möller, Thomas; Stella, Nephi (2004-03-02). "P2X7 receptors control 2-arachidonoylglycerol production by microglial cells". Proceedings of the National Academy of Sciences of the United States of America. 101 (9): 3214–3219. Bibcode:2004PNAS..101.3214W. doi:10.1073/pnas.0306707101. ISSN 0027-8424. PMC 365769. PMID 14976257.
- ^ Kondo S, Kondo H, Nakane S, et al. (June 1998). "2-Arachidonoylglycerol, an endogenous cannabinoid receptor agonist: identification as one of the major species of monoacylglycerols in various rat tissues, and evidence for its generation through Ca2+-dependent and -independent mechanisms". FEBS Letters. 429 (2): 152–6. doi:10.1016/S0014-5793(98)00581-X. PMID 9650580. S2CID 10583431.
- ^ Fride E, Bregman T, Kirkham TC (April 2005). "Endocannabinoids and food intake: newborn suckling and appetite regulation in adulthood" (PDF). Experimental Biology and Medicine. 230 (4): 225–234. doi:10.1177/153537020523000401. PMID 15792943. S2CID 25430588.
- ^ The Endocannabinoid-CB Receptor System: Importance for development and in pediatric disease Neuroendocrinology Letters Nos.1/2, Feb-Apr Vol.25, 2004.
- ^ Cannabinoids and Feeding: The Role of the Endogenous Cannabinoid System as a Trigger for Newborn Suckling Archived 2020-10-01 at the Wayback Machine Women and Cannabis: Medicine, Science, and Sociology, 2002 The Haworth Press, Inc.
- ^ Pizzorno, Lara; MDiv; MA; LMT. "New Developments in Cannabinoid-Based Medicine: An Interview with Dr. Raphael Mechoulam" Archived 2018-06-19 at the Wayback Machine. Longevity Medicine Review. Retrieved 2011-05-26.
- ^ Sugiura T, Itoh K, Waku K, Hanahan DJ (1994) Proceedings of Japanese conference on the Biochemistry of Lipids, 36, 71-74 (in Japanese)
- ^ Sugiura T, Kondo S, Sukagawa A, et al. (October 1995). "2-Arachidonoylglycerol: a possible endogenous cannabinoid receptor ligand in brain". Biochem. Biophys. Res. Commun. 215 (1): 89–97. doi:10.1006/bbrc.1995.2437. PMID 7575630.
- ^ Mechoulam R, Ben-Shabat S, Hanuš L, et al. (June 1995). "Identification of an endogenous 2-monoglyceride, present in canine gut, that binds to cannabinoid receptors". Biochemical Pharmacology. 50 (1): 83–90. doi:10.1016/0006-2952(95)00109-D. PMID 7605349.
- ^ Marzo, Vincenzo Di (2004). Cannabinoids (Neuroscience Intelligence Unit) (1st ed.). Georgetown, Texas: Springer. pp. 99, 181. ISBN 978-0-306-48228-1.
- ^ Savinainen JR, Järvinen T, Laine K, Laitinen JT (October 2001). "Despite substantial degradation, 2-arachidonoylglycerol is a potent full efficacy agonist mediating CB(1) receptor-dependent G-protein activation in rat cerebellar membranes". British Journal of Pharmacology. 134 (3): 664–72. doi:10.1038/sj.bjp.0704297. PMC 1572991. PMID 11588122.
- ^ Miller, Melissa R.; Mannowetz, Nadja; Iavarone, Anthony T.; Safavi, Rojin; Gracheva, Elena O.; Smith, James F.; Hill, Rose Z.; Bautista, Diana M.; Kirichok, Yuriy; Lishko, Polina V. (2016-04-29). "Unconventional endocannabinoid signaling governs sperm activation via the sex hormone progesterone". Science. 352 (6285): 555–559. Bibcode:2016Sci...352..555M. doi:10.1126/science.aad6887. ISSN 0036-8075. PMC 5373689. PMID 26989199.
- ^ Blankman JL, Simon GM, Cravatt BF (December 2007). "A comprehensive profile of brain enzymes that hydrolyze the endocannabinoid 2-arachidonoylglycerol". Chemistry & Biology. 14 (12): 1347–56. doi:10.1016/j.chembiol.2007.11.006. PMC 2692834. PMID 18096503.
- ^ Savinainen, JR; Saario, SM; Laitinen, JT (2012). "The serine hydrolases MAGL, ABHD6 and ABHD12 as guardians of 2-arachidonoylglycerol signalling through cannabinoid receptors". Acta Physiologica. 204 (2): 267–76. doi:10.1111/j.1748-1716.2011.02280.x. PMC 3320662. PMID 21418147.
- ^ Kaczocha, M.; Glaser, S.T.; Deutsch, D.G. (2009). "Identification of intracellular carriers for the endocannabinoid anandamide". Proceedings of the National Academy of Sciences of the United States of America. 106 (15): 6375–6380. Bibcode:2009PNAS..106.6375K. doi:10.1073/pnas.0901515106. PMC 2669397. PMID 19307565.
- ^ Oddi, S.; Fezza, F.; Pasquariello, N.; d'Agostino, A.; Catanzaro, G.; De Simone, C.; Rapino, C.; Finazzi-Agrò, A.; MacCarrone, M. (2009). "Molecular identification of albumin and Hsp70 as cytosolic anandamide-binding proteins". Chemistry & Biology. 16 (6): 624–632. doi:10.1016/j.chembiol.2009.05.004. PMID 19481477.
- ^ Murataeva N, Straiker A, Mackie K (Mar 2014). "Parsing the players: 2-arachidonoylglycerol synthesis and degradation in the CNS". Br J Pharmacol. 171 (6): 1379–91. doi:10.1111/bph.12411. PMC 3954479. PMID 24102242.
General references
- Dinh TP, Carpenter D, Leslie FM, et al. (August 2002). "Brain monoglyceride lipase participating in endocannabinoid inactivation". Proceedings of the National Academy of Sciences of the United States of America. 99 (16): 10819–24. Bibcode:2002PNAS...9910819D. doi:10.1073/pnas.152334899. PMC 125056. PMID 12136125.
- Webarchive template wayback links
- Articles without KEGG source
- Articles containing unverified chemical infoboxes
- Chembox image size set
- Articles with short description
- Short description matches Wikidata
- Endocannabinoids
- Neurotransmitters
- Fatty acid esters
- CB1 receptor agonists
- Glycine receptor antagonists
- Glycerol esters
- Arachidonyl compounds