Cenobamate
 | |
| Names | |
|---|---|
| Trade names | Xcopri, Ontozry |
| Other names | YKP3089 |
| |
| Clinical data | |
| Main uses | Focal-onset seizures[1] |
| Side effects | Sleepiness, dizziness, tiredness, double vision, headache[1] |
| Routes of use | By mouth |
| External links | |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a620021 |
| Legal | |
| License data |
|
| Legal status |
|
| Pharmacokinetics | |
| Bioavailability | ≥88% |
| Protein binding | 60% |
| Metabolism | Mainly glucuronidation via UGT2B7 |
| Elimination half-life | 50–60 hours |
| Excretion | Mainly via urine |
| Chemical and physical data | |
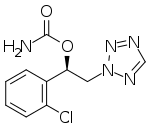
| Formula | C10H10ClN5O2 |
| Molar mass | 267.67 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Cenobamate, sold under the brand name Xcopri and Ontozry, is a medication used to treat epilepsy, specifically focal-onset seizures.[1] It may be used together with other medication.[2] It is taken by mouth.[1]
Common side effects include sleepiness, dizziness, tiredness, double vision, and headache.[1] Other side effects may include short QT, DRESS, and suicide.[1] Use in pregnancy may harm the baby.[1] How it works is not entirely clear; however, it alters ion channels.[1]
Cenobamate was approved for medical use in the United States in 2019 and Europe in 2021.[4][2] It is on Schedule V of the Controlled Substances Act.[5] In the United States 30 pills of 100 mg costs about 1,000 USD as of 2022.[6]
Medical uses
In the United States, cenobamate is indicated for the treatment of partial-onset seizures in adults.[1]
In the European Union, it is indicated for the adjunctive treatment of focal-onset seizures with or without secondary generalization in adults with epilepsy who have not been adequately controlled despite a history of treatment with at least two anti-epileptic medications.[2]
Dosage
It is generally started at a dose of 12.5 mg once per day and than gradually increased to 200 mg once per day.[2] Up to 400 mg once per day may be used.[2]
Contraindications
Cenobamate shortens the QT interval of the heart rhythm. It is therefore contraindicated in people with familial short QT syndrome, a very rare disease of the electrical system of the heart.[7][8]
Side effects
The most common side effects are drowsiness (in 37% of people taking the drug), dizziness (33%), and fatigue (24%). Sight disorders, headache and hyperkalaemia (high potassium levels in the blood) are also common.[7] Hypersensitivity occurs in fewer than 1% of patients, drug reaction with eosinophilia and systemic symptoms (DRESS) in fewer than 0.1%.[8]
Overdose
There are few data regarding cenobamate overdose. It is expected that the described adverse effects such as drowsiness, dizziness and fatigue would occur, as well as possibly problems with the heart rhythm. No specific antidote exists.[7][8]
Interactions
Using cenobamate together with other central nervous system depressants such as barbiturates, benzodiazepines or alcohol may result in increased drowsiness and other central nervous system symptoms.[7][8]
Cenobamate induces the enzymes CYP3A4 and CYP2B6 and can therefore decrease blood concentrations of drugs that are metabolized by these enzymes (for example midazolam and bupropion, respectively). Conversely, it inhibits the enzyme CYP2C19, potentially increasing concentrations of drugs metabolized by this enzyme (for example omeprazole).[7][8]
Pharmacology
Mechanism of action
Cenobamate is a voltage-gated sodium channel (VGSC) blocker.[9] It is a selective blocker of the inactivated state of VGSCs, preferentially inhibiting persistent sodium current.[9] It has been proposed that cenobamate additionally enhances presynaptic release of γ-aminobutyric acid (GABA), thereby increasing inhibitory GABAergic neurotransmission.[9]
Pharmacokinetics
Cenobamate is absorbed from the gut to at least 88% and reaches highest concentrations in the blood plasma after one to four hours. When in the bloodstream, 60% of the substance are bound to plasma proteins, mostly to albumin. Cenobamate is inactivated mainly by glucuronidation via the enzyme UGT2B7 and to a lesser extent UGT2B4. The enzymes CYP2E1, CYP2A6, CYP2B6, CYP2C19 and CYP3A4 play smaller roles in the drug's metabolism.[8]
Steady state conditions are reached after 14 days. Cenobamate and its metabolites are mostly eliminated via the urine and only to 5.2% via the faeces. The terminal half-life is 50 to 60 hours.[8]
History
The safety and efficacy of cenobamate to treat partial-onset seizures was established in two randomized, double-blind, placebo-controlled studies that enrolled 655 adults. In these studies, patients had partial-onset seizures with or without secondary generalization for an average of approximately 24 years and median seizure frequency of 8.5 seizures per 28 days during an 8-week baseline period. During the trials, doses of 100, 200, and 400 milligrams (mg) daily reduced the number of seizures per 28 days compared with the placebo group.[10]
Society and culture
Legal status
The U.S. Food and Drug Administration (FDA) approved cenobamate in November 2019, and granted the application for Xcopri to SK Life Science Inc.[10][11][12][13]
On 28 January 2021, the Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency (EMA) adopted a positive opinion, recommending the granting of a marketing authorization.[14] The applicant for this medicinal product is Arvelle Therapeutics Netherlands B.V.[14] Ontozry was approved on 26 March 2021.[2][15]
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 1.6 1.7 1.8 1.9 "Xcopri Titration Pack- cenobamate kit Xcopri- cenobamate tablet, film coated Xcopri Maintenance Pack- cenobamate kit". DailyMed. Archived from the original on 11 August 2020. Retrieved 1 February 2021.
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 2.6 "Ontozry EPAR". European Medicines Agency (EMA). 25 January 2021. Archived from the original on 4 June 2021. Retrieved 4 June 2021. Text was copied from this source which is © European Medicines Agency. Reproduction is authorized provided the source is acknowledged.
- ↑ "Schedules of Controlled Substances: Placement of Cenobamate in Schedule V". Federal Register. 10 March 2020. Archived from the original on 3 April 2021. Retrieved 4 August 2021.
- ↑ "Cenobamate Monograph for Professionals". Drugs.com. Archived from the original on 29 November 2022. Retrieved 3 January 2022.
- ↑ "2020 - Placement of Cenobamate in Schedule V". DEA Diversion Control Division. 10 March 2020. Archived from the original on 19 April 2020. Retrieved 11 March 2020.
- ↑ "Xcopri Prices, Coupons & Savings Tips - GoodRx". GoodRx. Archived from the original on 19 October 2021. Retrieved 3 January 2022.
- ↑ 7.0 7.1 7.2 7.3 7.4 Xcopri FDA Professional Drug Information. Accessed 2021-07-28.
- ↑ 8.0 8.1 8.2 8.3 8.4 8.5 8.6 "Ontozry: EPAR – Product information" (PDF). European Medicines Agency. 2 June 2021. Archived (PDF) from the original on 28 July 2021. Retrieved 4 August 2021.
- ↑ 9.0 9.1 9.2 Younus I, Reddy DS (January 2018). "A resurging boom in new drugs for epilepsy and brain disorders". Expert Review of Clinical Pharmacology. 11 (1): 27–45. doi:10.1080/17512433.2018.1386553. PMID 28956955. S2CID 5598471.
- ↑ 10.0 10.1 "FDA approves new treatment for adults with partial-onset seizures". U.S. Food and Drug Administration (FDA) (Press release). 21 November 2019. Archived from the original on 22 November 2019. Retrieved 21 November 2019.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ↑ "Drug Trials Snapshots: Xcopri". U.S. Food and Drug Administration (FDA). 3 December 2019. Archived from the original on 19 December 2019. Retrieved 18 December 2019.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ↑ "Drug Approval Package: Xcopri". U.S. Food and Drug Administration (FDA). 10 December 2019. Archived from the original on 19 December 2019. Retrieved 18 December 2019.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ↑ "Cenobamate FDA Approval Status". Drugs.com. 13 November 2019. Retrieved 22 November 2019.
- ↑ 14.0 14.1 "Ontozry: Pending EC decision". European Medicines Agency (EMA). 29 January 2021. Archived from the original on 1 February 2021. Retrieved 1 February 2021. Text was copied from this source which is © European Medicines Agency. Reproduction is authorized provided the source is acknowledged.
- ↑ "Ontozry". Union Register of medicinal products. Archived from the original on 2 August 2021. Retrieved 3 August 2021.
External links
| Identifiers: |
|
|---|
- "Cenobamate". Drug Information Portal. U.S. National Library of Medicine (NLM). Archived from the original on 1 November 2021. Retrieved 4 August 2021.
- Pages using duplicate arguments in template calls
- Wikipedia articles incorporating the PD-notice template
- Use dmy dates from November 2019
- Articles with invalid date parameter in template
- Drugs with non-standard legal status
- Chemical articles with unknown parameter in Infobox drug
- Chemical articles without CAS registry number
- Articles without EBI source
- Chemical pages without ChemSpiderID
- Chemical pages without DrugBank identifier
- Articles without KEGG source
- Articles without UNII source
- Drugs missing an ATC code
- Drug has EMA link
- Articles containing unverified chemical infoboxes
- Anticonvulsants
- Carbamates
- Chloroarenes
- Tetrazoles
- RTT