Glibenclamide
 | |
 | |
| Names | |
|---|---|
| Trade names | Diabeta, Flycron, others[1] |
| Other names | Glyburide (USAN US) |
| |
| Clinical data | |
| Drug class | Sulfonylurea |
| Main uses | Type 2 diabetes[1] |
| Side effects | Nausea, heartburn, angioedema, low blood sugar[1] |
| Pregnancy category |
|
| Routes of use | By mouth |
| Defined daily dose | 7 to 10 mg[2] |
| External links | |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a684058 |
| Legal | |
| License data | |
| Legal status | |
| Pharmacokinetics | |
| Protein binding | Extensive |
| Metabolism | Liver hydroxylation (CYP2C9-mediated) |
| Elimination half-life | 10 hours |
| Excretion | Kidney and biliary |
| Chemical and physical data | |
| Formula | C23H28ClN3O5S |
| Molar mass | 494.00 g·mol−1 |
| 3D model (JSmol) | |
| Melting point | 169 to 170 °C (336 to 338 °F) |
| |
| |
Glibenclamide, also known as glyburide, is a medication used to treat type 2 diabetes.[1] It is recommended that it be taken together with diet and exercise.[1] It may be used with other antidiabetic medication.[1] It is not recommended for use by itself in diabetes mellitus type 1.[1] It is taken by mouth.[1]
Common side effects include nausea and heartburn.[1] Serious side effects may include angioedema and low blood sugar.[1] It is generally not recommended during pregnancy but can be used during breastfeeding.[3] It is in the sulfonylurea class of medications and works by increasing the release of insulin from the pancreas.[1]
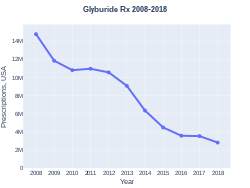
Glibenclamide was discovered in 1969 and approved for medical use in the United States in 1984.[4][1] It is available as a generic medication.[3] A month supply in the United Kingdom costs the NHS about £3.20 as of 2019.[3] In the United States the wholesale cost of this amount is about $2.50.[5] In 2017, it was the 174th most commonly prescribed medication in the United States, with more than three million prescriptions.[6][7]
Medical uses
It is used in the treatment of type 2 diabetes.
It is not as good as either metformin or insulin in those who have gestational diabetes.[8]
Dosage
The defined daily dose is 7 to 10 mg by mouth depending on the formulation.[2] In adults it is generally started at 2.5 mg per day for the first week and than increased to 5 mg per day for the second week.[9] The typical dose is 5 mg twice per day with a maximum dose of 15 mg in a day.[9]
Side effects
Frequently reported side effects include: nausea, heartburn, weight gain, and bloating.[10] The medication is also a major cause of medication-induced hypoglycemia. The risk is greater than with other sulfonylureas.[11] Cholestatic jaundice is noted.
Glibenclamide may be not recommended in those with G6PD deficiency, as it may cause acute hemolysis.[12]
Pregnancy and breastfeeding
It is generally not recommended during pregnancy but can be used during breastfeeding.[3]
Mechanism of action
The medication works by binding to and inhibiting the ATP-sensitive potassium channels (KATP) inhibitory regulatory subunit sulfonylurea receptor 1 (SUR1)[13] in pancreatic beta cells. This inhibition causes cell membrane depolarization, opening voltage-dependent calcium channels. This results in an increase in intracellular calcium in the pancreatic beta cell and subsequent stimulation of insulin release.
After a cerebral ischemic insult, the blood–brain barrier is broken and glibenclamide can reach the central nervous system. Glibenclamide has been shown to bind more efficiently to the ischemic hemisphere.[14] Moreover, under ischemic conditions SUR1, the regulatory subunit of the KATP- and the NCCa-ATP-channels, is expressed in neurons, astrocytes, oligodendrocytes, endothelial cells[15] and by reactive microglia.[14]
History
It was developed in 1966 in a cooperative study between Boehringer Mannheim (now part of Roche) and Hoechst (now part of Sanofi-Aventis).[16]
Trade names
Glibenclamide is available as a generic, is manufactured by many pharmaceutical companies and is sold in doses of 1.25, 2.5 and 5 mg under many brand names including Gliben-J, Daonil, Diabeta, Euglucon, Gilemal, Glidanil, Glybovin, Glynase, Maninil, Micronase and Semi-Daonil. It is also available in a fixed-dose combination drug with metformin that is sold under various trade names, e.g. Bagomet Plus, Benimet, Glibomet, Gluconorm, Glucored, Glucovance, Metglib and many others.[citation needed]
Society and culture
Cost
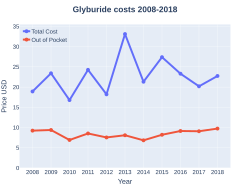
A month supply in the United Kingdom costs the NHS about £3.20 as of 2019.[3] In the United States the wholesale cost of this amount is about $2.50.[5] In 2017, it was the 174th most commonly prescribed medication in the United States, with more than three million prescriptions.[6][7]
-
Glyburide costs (US)
-
Glyburide prescriptions (US)
References
- ↑ 1.00 1.01 1.02 1.03 1.04 1.05 1.06 1.07 1.08 1.09 1.10 1.11 "Glyburide Monograph for Professionals". Drugs.com. American Society of Health-System Pharmacists. Archived from the original on 31 March 2019. Retrieved 18 March 2019.
- ↑ 2.0 2.1 "WHOCC - ATC/DDD Index". www.whocc.no. Archived from the original on 21 January 2021. Retrieved 30 August 2020.
- ↑ 3.0 3.1 3.2 3.3 3.4 British national formulary : BNF 76 (76 ed.). Pharmaceutical Press. 2018. p. 692. ISBN 9780857113382.
- ↑ Diabetes in Clinical Practice: Questions and Answers from Case Studies. John Wiley & Sons. 2007. p. 342. ISBN 9780470059135. Archived from the original on 2019-03-31. Retrieved 2019-03-19.
- ↑ 5.0 5.1 "NADAC as of 2019-02-27". Centers for Medicare and Medicaid Services. Archived from the original on 2019-03-06. Retrieved 3 March 2019.
- ↑ 6.0 6.1 "The Top 300 of 2020". ClinCalc. Archived from the original on 12 February 2021. Retrieved 11 April 2020.
- ↑ 7.0 7.1 "Glyburide - Drug Usage Statistics". ClinCalc. Archived from the original on 12 April 2020. Retrieved 11 April 2020.
- ↑ Balsells, M; García-Patterson, A; Solà, I; Roqué, M; Gich, I; Corcoy, R (21 January 2015). "Glibenclamide, metformin, and insulin for the treatment of gestational diabetes: a systematic review and meta-analysis". BMJ (Clinical Research Ed.). 350: h102. doi:10.1136/bmj.h102. PMC 4301599. PMID 25609400.
- ↑ 9.0 9.1 "GLIBENCLAMIDE oral - Essential drugs". medicalguidelines.msf.org. Archived from the original on 28 August 2021. Retrieved 30 August 2020.
- ↑ "Glyburide: MedlinePlus Drug Information". medlineplus.gov. Archived from the original on 14 April 2019. Retrieved 29 October 2019.
- ↑ Gangji, A. S.; Cukierman, T.; Gerstein, H. C.; Goldsmith, C. H.; Clase, C. M. (1 February 2007). "A Systematic Review and Meta-Analysis of Hypoglycemia and Cardiovascular Events: A comparison of glyburide with other secretagogues and with insulin". Diabetes Care. 30 (2): 389–394. doi:10.2337/dc06-1789. PMID 17259518.
- ↑ Meloni G, Meloni T (January 1996). "Glyburide-induced acute haemolysis in a G6PD-deficient patient with NIDDM". Br. J. Haematol. 92 (1): 159–60. doi:10.1046/j.1365-2141.1996.275810.x. PMID 8562390.
- ↑ Serrano-Martín X, Payares G, Mendoza-León A (December 2006). "Glibenclamide, a blocker of K+(ATP) channels, shows antileishmanial activity in experimental murine cutaneous leishmaniasis". Antimicrob. Agents Chemother. 50 (12): 4214–6. doi:10.1128/AAC.00617-06. PMC 1693980. PMID 17015627.
- ↑ 14.0 14.1 Ortega FJ, Gimeno-Bayon J, Espinosa-Parrilla JF, Carrasco JL, Batlle M, Pugliese M, Mahy N, Rodríguez MJ (May 2012). "ATP-dependent potassium channel blockade strengthens microglial neuroprotection after hypoxia-ischemia in rats" (PDF). Exp. Neurol. 235 (1): 282–96. doi:10.1016/j.expneurol.2012.02.010. hdl:2445/34278. PMID 22387180. Archived (PDF) from the original on 2021-06-06. Retrieved 2019-09-24.
- ↑ Simard JM, Woo SK, Schwartzbauer GT, Gerzanich V (September 2012). "Sulfonylurea receptor 1 in central nervous system injury: a focused review". J. Cereb. Blood Flow Metab. 32 (9): 1699–717. doi:10.1038/jcbfm.2012.91. PMC 3434627. PMID 22714048.
- ↑ Marble A (1971). "Glibenclamide, a new sulphonylurea: whither oral hypoglycaemic agents?". Drugs. 1 (2): 109–15. doi:10.2165/00003495-197101020-00001. PMID 4999930.
External links
| Identifiers: |
|
|---|
- "Glyburide". Drug Information Portal. U.S. National Library of Medicine. Archived from the original on 2020-10-18. Retrieved 2020-05-16.
- Pages using duplicate arguments in template calls
- Infobox drug with local INN variant
- Chemical articles with unknown parameter in Infobox drug
- Chemical articles without CAS registry number
- Articles without EBI source
- Chemical pages without ChemSpiderID
- Chemical pages without DrugBank identifier
- Articles without KEGG source
- Articles without UNII source
- Drugs missing an ATC code
- Drugboxes which contain changes to watched fields
- All articles with unsourced statements
- Articles with unsourced statements from January 2018
- Articles with invalid date parameter in template
- Disulfiram-like drugs
- Potassium channel blockers
- Benzamides
- Chloroarenes
- Phenol ethers
- 1-(Benzenesulfonyl)-3-cyclohexylureas
- Sanofi
- RTT