Glipizide
 | |
 | |
| Names | |
|---|---|
| Trade names | Glucotrol, Glucotrol XL, others |
| |
| Clinical data | |
| Drug class | Sulfonylurea |
| Main uses | Type 2 diabetes[1] |
| Side effects | Diarrhea, low blood sugar, headache[1] |
| Pregnancy category |
|
| Routes of use | By mouth |
| Defined daily dose | 10 mg[2] |
| External links | |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a684060 |
| Legal | |
| Legal status | |
| Pharmacokinetics | |
| Bioavailability | 100% (regular formulation) 90% (extended release) |
| Protein binding | 98 to 99% |
| Metabolism | Liver hydroxylation |
| Elimination half-life | 2 to 5 hours |
| Excretion | Kidney and fecal |
| Chemical and physical data | |
| Formula | C21H27N5O4S |
| Molar mass | 445.54 g·mol−1 |
| 3D model (JSmol) | |
| Melting point | 208 to 209 °C (406 to 408 °F) |
| |
| |
Glipizide, sold under the brand name Glucotrol among others, is an anti-diabetic medication of the sulfonylurea class used to treat type 2 diabetes.[1] It is used together with a diabetic diet and exercise.[1][3] It is not indicated for use by itself in type 1 diabetes.[1] It is taken by mouth.[1] Effects generally begin within half an hour and can last for up to a day.[1]
Common side effects include nausea, diarrhea, low blood sugar, and headache.[1] Other side effects include sleepiness, skin rash, and shakiness.[4] The dose may need to be adjusted in those with liver or kidney disease.[1] Use during pregnancy or breastfeeding is not recommended.[4] It works by stimulating the pancreas to release insulin and increases tissue sensitivity to insulin.[1]
Glipizide was approved for medical use in the United States in 1984.[1] It is available as a generic medication.[1] In the United States the wholesale cost per dose is less than US$0.05 as of 2018.[5] In the United Kingdom it costs the NHS less than GB£0.05 per dose as of 2018.[4] In 2017, it was the 45th most commonly prescribed medication in the United States, with more than 16 million prescriptions.[6][7]
Medical uses
Dosage
The defined daily dose is 10 mg by mouth.[2]
Mechanism of action
Glipizide sensitizes the beta cells of pancreatic islets of Langerhans insulin response, meaning that more insulin is released in response to glucose than would be without glipizide ingestion.[3] Glipizide acts by partially blocking potassium channels among beta cells of pancreatic islets of Langerhans. By blocking potassium channels, the cell depolarizes, which results in the opening of voltage-gated calcium channels. The resulting calcium influx encourages insulin release from beta cells.[8]
History
It was patented in 1969 and approved for medical use in 1971.[9] Glipizide was approved for medical use in the United States in 1984.[1]
Society and culture
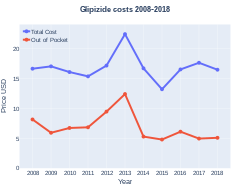
Cost
In the United States the wholesale cost per dose is less than US$0.05 as of 2018.[5] In the United Kingdom it costs the NHS less than GB£0.05 per dose as of 2018.[4] In 2017, it was the 45th most commonly prescribed medication in the United States, with more than 16 million prescriptions.[6][7]
-
Glipizide costs (US)
-
Glipizide prescriptions (US)
See also
References
- ↑ 1.00 1.01 1.02 1.03 1.04 1.05 1.06 1.07 1.08 1.09 1.10 1.11 1.12 "Glipizide Monograph for Professionals". Drugs.com. AHFS. Archived from the original on 13 January 2020. Retrieved 24 December 2018.
- ↑ 2.0 2.1 "WHOCC - ATC/DDD Index". www.whocc.no. Archived from the original on 2021-02-25. Retrieved 2020-09-09.
- ↑ 3.0 3.1 "Glucotrol XL- glipizide tablet, extended release". DailyMed. 17 August 2018. Archived from the original on 16 February 2017. Retrieved 31 July 2020.
- ↑ 4.0 4.1 4.2 4.3 British national formulary : BNF 76 (76 ed.). Pharmaceutical Press. 2018. p. 693. ISBN 9780857113382.
- ↑ 5.0 5.1 "NADAC as of 2018-12-19". Centers for Medicare and Medicaid Services. Archived from the original on 2018-12-19. Retrieved 22 December 2018.
- ↑ 6.0 6.1 "The Top 300 of 2020". ClinCalc. Archived from the original on 12 February 2021. Retrieved 11 April 2020.
- ↑ 7.0 7.1 "Glipizide - Drug Usage Statistics". ClinCalc. Archived from the original on 12 April 2020. Retrieved 11 April 2020.
- ↑ Bösenberg LH, Van Zyl DG (December 2008). "The mechanism of action of oral antidiabetic drugs: a review of recent literature". Journal of Endocrinology, Metabolism and Diabetes of South Africa. 13 (3): 80–8. doi:10.1080/22201009.2008.10872177.
- ↑ Fischer, Jnos; Ganellin, C. Robin (2006). Analogue-based Drug Discovery. John Wiley & Sons. p. 449. ISBN 9783527607495. Archived from the original on 2016-12-27. Retrieved 2020-07-31.
External links
| External sites: | |
|---|---|
| Identifiers: |
|
- Pages using duplicate arguments in template calls
- Chemical articles with unknown parameter in Infobox drug
- Chemical articles without CAS registry number
- Articles without EBI source
- Chemical pages without ChemSpiderID
- Chemical pages without DrugBank identifier
- Articles without KEGG source
- Articles without UNII source
- Drugs missing an ATC code
- Drugboxes which contain changes to verified fields
- Drugboxes which contain changes to watched fields
- Potassium channel blockers
- 1-(Benzenesulfonyl)-3-cyclohexylureas
- Pyrazines
- Pfizer brands
- RTT