Ibuprofen
 | |
 | |
| Names | |
|---|---|
| Pronunciation | /ˈaɪbjuːproʊfɛn/, /aɪbjuːˈproʊfən/, EYE-bew-PROH-fən |
| Trade names | Advil, Motrin, Nurofen, others |
| Other names | isobutylphenylpropionic acid |
| |
| Clinical data | |
| Drug class | Nonsteroidal anti-inflammatory drug (NSAID) |
| Main uses | Pain, fever, inflammation[1] |
| Side effects | Heartburn, rash[1] |
| Pregnancy category |
|
| Breastfeeding | Safe[3] |
| Routes of use | By mouth, rectal, topical, intravenous |
| Onset of action | 30 min[4] |
| Defined daily dose | 1.2 g (by mouth) or 1.2 g (parenteral) or 1.2 g (rectal) for pain relief[5] and 30 mg (parenteral) for treatment of ischemic heart diseases[6] |
| External links | |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a682159 |
| Legal | |
| License data | |
| Legal status |
|
| Pharmacokinetics | |
| Bioavailability | 80–100% (by mouth),[7] 87% (rectal) |
| Protein binding | 98%[8] |
| Metabolism | Liver (CYP2C9)[8] |
| Metabolites | ibuprofen glucuronide, 2-hydroxyibuprofen, 3-hydroxyibuprofen, carboxy-ibuprofen, 1-hydroxyibuprofen |
| Elimination half-life | 2–4 h[9] |
| Excretion | Urine (95%)[8][10] |
| Chemical and physical data | |
| Formula | C13H18O2 |
| Molar mass | 206.285 g·mol−1 |
| 3D model (JSmol) | |
| Chirality | Racemic mixture |
| Density | 1.03 g/ml g/cm3 |
| Melting point | 75 to 78 °C (167 to 172 °F) |
| Boiling point | 157 °C (315 °F) at 4 mmHg |
| Solubility in water | 0.021 mg/mL (20 °C) |
| |
| |
Ibuprofen is a medication in the nonsteroidal anti-inflammatory drug (NSAID) class that is used for treating pain, fever, and inflammation.[1] This includes painful menstrual periods, migraines, and rheumatoid arthritis.[1] It may also be used to close a patent ductus arteriosus in a premature baby.[1] It can be used by mouth or intravenously.[1] It typically begins working within an hour.[1]
Common side effects include heartburn and a rash.[1] Compared to other NSAIDs, it may have fewer side effects such as gastrointestinal bleeding.[11] It increases the risk of heart failure, kidney failure, and liver failure.[1] At low doses, it does not appear to increase the risk of heart attack; however, at higher doses it may.[11] Ibuprofen can also worsen asthma.[11] While it is unclear if it is safe in early pregnancy,[1] it appears to be harmful in later pregnancy and therefore is not recommended.[12] Use when breastfeeding is safe.[3] Like other NSAIDs, it works by inhibiting the production of prostaglandins by decreasing the activity of the enzyme cyclooxygenase.[1] Ibuprofen is a weaker anti-inflammatory agent than other NSAIDs.[11]
Ibuprofen was discovered in 1961 by Stewart Adams at Boots UK Limited and initially marketed as Brufen.[13] It is available under a number of trade names, including Nurofen, Advil and Motrin.[1][14] It was first marketed in 1969 in the United Kingdom and in the United States in 1974.[1][13] It is on the World Health Organization's List of Essential Medicines.[15] It is available as a generic medication.[1] The wholesale cost in the developing world is between US$0.01 and US$0.04 per dose.[16] In the United States, it costs about US$0.05 per dose.[1] In 2017, it was the 28th most commonly prescribed medication in the United States, with more than 24 million prescriptions.[17][18]
Medical uses

Ibuprofen is used primarily to treat fever (including post-vaccination fever), mild to moderate pain (including pain relief after surgery), painful menstruation, osteoarthritis, dental pain, headaches, and pain from kidney stones. About 60% of people respond to any NSAID; those who do not respond well to a particular one may respond to another.[19]
It is used for inflammatory diseases such as juvenile idiopathic arthritis and rheumatoid arthritis.[20][21] It is also used for pericarditis and patent ductus arteriosus.[22][23]
In children under the age of two it works better than acetaminophen for fever or pain with similar safety.[24]
Ibuprofen lysine
In some countries, ibuprofen lysine (the lysine salt of ibuprofen, sometimes called "ibuprofen lysinate") is used for the same conditions as ibuprofen; the lysine salt is used because it is more water-soluble.[25] In 2006, ibuprofen lysine was approved in the USA for closure of patent ductus arteriosus in premature infants weighing between 500 and 1,500 grams (1 and 3 lb), who are no more than 32 weeks' gestational age when usual medical management (such as fluid restriction, diuretics, and respiratory support) is not effective.[26]
Dosage
The defined daily dose is 1.2 g (by mouth) or 1.2 g (parenteral) or 1.2 g (rectal) for pain relief[27] and 30 mg (parenteral) for treatment of ischemic heart diseases[6] In those over the age of 12 the dose is generally 200 to 400 mg three to four times a day while in those over the age of three months the dose is 5 to 10 mg/kg three to four times per day.[28] In rheumatologic diseases doses up to 40 mg/kg per day in children or 3,200 mg per day in adults may be used.[28]
Side effects
Side effects include nausea, dyspepsia, diarrhea, constipation, gastrointestinal ulceration/bleeding, headache, dizziness, rash, salt and fluid retention, and high blood pressure.[21][29]
Infrequent adverse effects include esophageal ulceration, heart failure, high blood levels of potassium, kidney impairment, confusion, and bronchospasm.[21] Ibuprofen can exacerbate asthma, sometimes fatally.[30]
Ibuprofen may be quantified in blood, plasma, or serum to demonstrate the presence of the drug in a person having experienced an anaphylactic reaction, confirm a diagnosis of poisoning in people who are hospitalized, or assist in a medicolegal death investigation. A monograph relating ibuprofen plasma concentration, time since ingestion, and risk of developing renal toxicity in people who have overdosed has been published.[31]
Cardiovascular risk
Along with several other NSAIDs, chronic ibuprofen use has been found correlated with risk of progression to hypertension in women, though less than for acetaminophen,[32] and myocardial infarction (heart attack),[33] particularly among those chronically using higher doses. On 9 July 2015, the U.S. Food and Drug Administration (FDA) toughened warnings of increased heart attack and stroke risk associated with ibuprofen and related NSAIDs; the NSAID aspirin is not included in this warning.[34] The European Medicines Agency (EMA) issued similar warnings in 2015.[35][36]
Skin
Along with other NSAIDs, ibuprofen has been associated with the onset of bullous pemphigoid or pemphigoid-like blistering.[37] As with other NSAIDs, ibuprofen has been reported to be a photosensitising agent,[38] but it is considered a weak photosensitising agent compared to other members of the 2-arylpropionic acid class. Like other NSAIDs, ibuprofen is an extremely rare cause of the autoimmune disease Stevens–Johnson syndrome (SJS).[39][40] Ibuprofen is also an extremely rare cause of toxic epidermal necrolysis.[41]
Interactions
Alcohol
Drinking alcohol when taking ibuprofen may increase the risk of stomach bleeding.[42]
Aspirin
According to the Food and Drug Administration (FDA), "ibuprofen can interfere with the antiplatelet effect of low-dose aspirin, potentially rendering aspirin less effective when used for cardioprotection and stroke prevention." Allowing sufficient time between doses of ibuprofen and immediate-release (IR) aspirin can avoid this problem. The recommended elapsed time between a dose of ibuprofen and a dose of aspirin depends on which is taken first. It would be 30 minutes or more for ibuprofen taken after IR aspirin, and eight hours or more for ibuprofen taken before IR aspirin. However, this timing cannot be recommended for enteric-coated aspirin. But, if ibuprofen is taken only occasionally without the recommended timing, the reduction of the cardioprotection and stroke prevention of a daily aspirin regimen is minimal.[43]
Paracetamol
Ibuprofen combined with paracetamol is considered generally safe in children for short-term usage.[44]
Overdose
Ibuprofen overdose has become common since it was licensed for OTC use. Many overdose experiences are reported in the medical literature, although the frequency of life-threatening complications from ibuprofen overdose is low.[45] Human response in cases of overdose ranges from absence of symptoms to fatal outcome despite intensive-care treatment. Most symptoms are an excess of the pharmacological action of ibuprofen, and include abdominal pain, nausea, vomiting, drowsiness, dizziness, headache, ear ringing, and nystagmus. Rarely, more severe symptoms, such as gastrointestinal bleeding, seizures, metabolic acidosis, high blood levels of potassium, low blood pressure, slow heart rate, fast heart rate, atrial fibrillation, coma, liver dysfunction, acute kidney failure, cyanosis, respiratory depression, and cardiac arrest have been reported.[46] The severity of symptoms varies with the ingested dose and the time elapsed; however, individual sensitivity also plays an important role. Generally, the symptoms observed with an overdose of ibuprofen are similar to the symptoms caused by overdoses of other NSAIDs.
Correlation between severity of symptoms and measured ibuprofen plasma levels is weak. Toxic effects are unlikely at doses below 100 mg/kg, but can be severe above 400 mg/kg (around 150 tablets of 200 mg units for an average man);[47] however, large doses do not indicate the clinical course is likely to be lethal.[48] A precise lethal dose is difficult to determine, as it may vary with age, weight, and concomitant conditions of the individual person.
Treatment to address an ibuprofen overdose is based on how the symptoms present. In cases presenting early, decontamination of the stomach is recommended. This is achieved using activated charcoal; charcoal adsorbs the drug before it can enter the bloodstream. Gastric lavage is now rarely used, but can be considered if the amount ingested is potentially life-threatening, and it can be performed within 60 minutes of ingestion. Purposeful vomiting is not recommended.[49] The majority of ibuprofen ingestions produce only mild effects and the management of overdose is straightforward. Standard measures to maintain normal urine output should be instituted and kidney function monitored.[47] Since ibuprofen has acidic properties and is also excreted in the urine, forced alkaline diuresis is theoretically beneficial. However, because ibuprofen is highly protein-bound in the blood, the kidneys' excretion of unchanged drug is minimal. Forced alkaline diuresis is, therefore, of limited benefit.[50]
Miscarriage
A study of pregnant women suggests that those taking any type or amount of NSAIDs (including ibuprofen, diclofenac and naproxen) were 2.4 times more likely to miscarry than those not taking the medications.[51] However, an Israeli study found no increased risk of miscarriage in the group of mothers using NSAIDs.[52]
Pharmacology
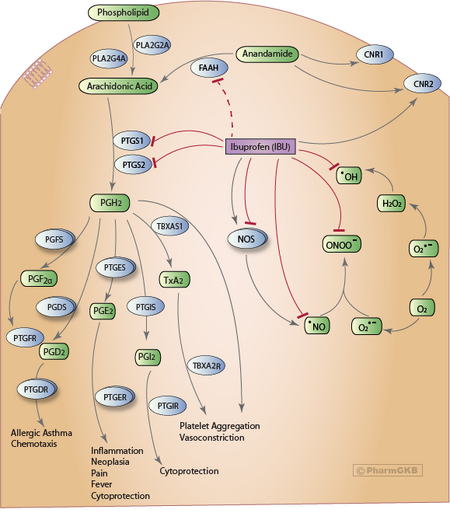
NSAIDs such as ibuprofen work by inhibiting the cyclooxygenase (COX) enzymes, which convert arachidonic acid to prostaglandin H2 (PGH2). PGH2, in turn, is converted by other enzymes to several other prostaglandins (which are mediators of pain, inflammation, and fever) and to thromboxane A2 (which stimulates platelet aggregation, leading to the formation of blood clots).
Like aspirin and indomethacin, ibuprofen is a nonselective COX inhibitor, in that it inhibits two isoforms of cyclooxygenase, COX-1 and COX-2. The analgesic, antipyretic, and anti-inflammatory activity of NSAIDs appears to operate mainly through inhibition of COX-2, which decreases the synthesis of prostaglandins involved in mediating inflammation, pain, fever, and swelling. Antipyretic effects may be due to action on the hypothalamus, resulting in an increased peripheral blood flow, vasodilation, and subsequent heat dissipation. Inhibition of COX-1 instead would be responsible for unwanted effects on the gastrointestinal tract.[54] However, the role of the individual COX isoforms in the analgesic, anti-inflammatory, and gastric damage effects of NSAIDs is uncertain and different compounds cause different degrees of analgesia and gastric damage.[55]
Ibuprofen is administered as a racemic mixture. The R-enantiomer undergoes extensive interconversion to the S-enantiomer in vivo. The S-enantiomer is believed to be the more pharmacologically active enantiomer.[56]
The R-enantiomer is converted through a series of three main enzymes. These enzymes include acyl-CoA-synthetase, which converts the R-enantiomer to (-)-R-ibuprofen I-CoA; 2-arylpropionyl-CoA epimerase, which converts (-)-R-ibuprofen I-CoA to (+)-S-Ibuprofen I-CoA; and hydrolase, which converts (+)-S-ibuprofen I-CoA to the S-enantiomer.[41] In addition to the conversion of ibuprofen to the S-enantiomer, the body can metabolize ibuprofen to several other compounds, including numerous hydroxyl, carboxyl and glucuronyl metabolites. Virtually all of these have no pharmacological effects.[41]
Pharmacokinetics
After oral administration, peak serum concentration is reached after 1–2 hours and up to 99% of the drug is bound to plasma proteins.[57] The majority of ibuprofen is metabolised and eliminated within 24 hours in the urine; however, 1% of the unchanged drug is removed through biliary excretion.[56]
Chemistry
 |
 |
 |
 |
Ibuprofen is practically insoluble in water, but very soluble in most organic solvents like ethanol (66.18 g/100mL at 40 °C for 90% EtOH), methanol, acetone and dichloromethane.[58]
The original synthesis of ibuprofen by the Boots Group started with the compound 2-methylpropylbenzene. The synthesis took six steps. A modern, greener technique for the synthesis involves only three steps.[59]
Stereochemistry
It is an optically active compound with both S and R-isomers, of which the S (dextrorotatory) isomer is the more biologically active; this isomer has also been isolated and used medically (see dexibuprofen for details).[58]
Ibuprofen is produced industrially as a racemate. The compound, like other 2-arylpropionate derivatives (including ketoprofen, flurbiprofen, naproxen, etc.), does contain a stereocenter in the α-position of the propionate moiety. So two enantiomers of ibuprofen occur, with the potential for different biological effects and metabolism for each enantiomer.
An isomerase (alpha-methylacyl-CoA racemase) converts (R)-ibuprofen to the active (S)-enantiomer.[60][61][62]
History

Ibuprofen was derived from propionic acid by the research arm of Boots Group during the 1960s.[63] The name is derived from the 3 functional groups: isobutyl (ibu) propionic acid (pro) phenyl (fen). Its discovery was the result of research during the 1950s and 1960s to find a safer alternative to aspirin.[13][64] The molecule was discovered and synthesized by a team led by Stewart Adams, with a patent application filed in 1961.[13] Adams initially tested the drug as treatment for his hangover.[65]
The drug was launched as a treatment for rheumatoid arthritis in the United Kingdom in 1969, and in the United States in 1974. Later, in 1983 and 1984, it became the first NSAID (other than aspirin) to be available over the counter (OTC) in these two countries.[13][64] Dr. Adams was subsequently awarded an Order of the British Empire (OBE) in 1987. Boots was awarded the Queen's Award for Technical Achievement in 1987 for the development of the drug.[13]
In November 2013, work on ibuprofen was recognized by the erection of a Royal Society of Chemistry blue plaque at Boots' Beeston Factory site in Nottingham, which reads:[66]
In recognition of the work during the 1980s by The Boots Company PLC on the development of ibuprofen which resulted in its move from prescription only status to over the counter sale, therefore expanding its use to millions of people worldwide
and another at BioCity Nottingham, the site of the original laboratory, which reads:[66]
In recognition of the pioneering research work, here on Pennyfoot Street, by Dr Stewart Adams and Dr John Nicholson in the Research Department of Boots which led to the discovery of ibuprofen used by millions worldwide for the relief of pain.
Society and culture
Cost
The wholesale cost in the developing world is between US$0.01 and US$0.04 per dose.[16] In the United States, it costs about US$0.05 per dose.[1] In 2017, it was the 28th most commonly prescribed medication in the United States, with more than 24 million prescriptions.[17][18]
-
Ibuprofen costs (US)
-
Ibuprofen prescriptions (US)
Availability

Ibuprofen was made available under prescription in the United Kingdom in 1969, and in the United States in 1974.[67] In the years since, the good tolerability profile, along with extensive experience in the population, as well as in so-called phase-IV trials (postapproval studies), have resulted in the availability of ibuprofen OTC in pharmacies worldwide, as well as in supermarkets and other general retailers.
Ibuprofen is its International nonproprietary name (INN), British Approved Name (BAN), Australian Approved Name (AAN) and United States Adopted Name (USAN). In the U.S., Motrin has been on the market since 1974,[68] and Advil has been on the market since 1984.[69] Ibuprofen is commonly available in the United States up to the FDA's 1984 dose limit OTC, rarely used higher by prescription.[70][failed verification]
In 2009, the first injectable formulation of ibuprofen was approved in the United States, under the trade name Caldolor.[71][72]
Route
It can be used by mouth, as a tablet, capsule or suspension, or intravenously.[1]
Research
Ibuprofen is sometimes used for the treatment of acne because of its anti-inflammatory properties, and has been sold in Japan in topical form for adult acne.[73][74] As with other NSAIDs, ibuprofen may be useful in the treatment of severe orthostatic hypotension (low blood pressure when standing up).[75] NSAIDs are of unclear utility in the prevention and treatment of Alzheimer's disease.[76][77]
Ibuprofen has been associated with a lower risk of Parkinson's disease, and may delay or prevent it. Aspirin, other NSAIDs, and paracetamol (acetaminophen) had no effect on the risk for Parkinson's.[78] In March 2011, researchers at Harvard Medical School announced in Neurology that ibuprofen had a neuroprotective effect against the risk of developing Parkinson's disease.[79][80][81] People regularly consuming ibuprofen were reported to have a 38% lower risk of developing Parkinson's disease, but no such effect was found for other pain relievers, such as aspirin and paracetamol. Use of ibuprofen to lower the risk of Parkinson's disease in the general population would not be problem-free, given the possibility of adverse effects on the urinary and digestive systems.[82]
Some dietary supplements might be dangerous to take along with ibuprofen and other NSAIDs, but as of 2016[update] more research needs to be conducted to be certain. These supplements include those that can prevent platelet aggregation, including ginkgo, garlic, ginger, bilberry, dong quai, feverfew, ginseng, turmeric, meadowsweet, and willow; those that contain coumarin, including chamomile, horse chestnut, fenugreek and red clover; and those that increase the risk of bleeding, like tamarind.[83]
References
- ↑ 1.00 1.01 1.02 1.03 1.04 1.05 1.06 1.07 1.08 1.09 1.10 1.11 1.12 1.13 1.14 1.15 1.16 "Ibuprofen". The American Society of Health-System Pharmacists. Archived from the original on 9 September 2017. Retrieved 12 October 2016.
- ↑ 2.0 2.1 2.2 Use During Pregnancy and Breastfeeding
- ↑ 3.0 3.1 "Ibuprofen use while Breastfeeding". Drugs.com. Archived from the original on 17 April 2021. Retrieved 29 March 2021.
- ↑ "ibuprofen". Archived from the original on 13 January 2015. Retrieved 31 January 2015.
- ↑ "WHOCC - ATC/DDD Index". www.whocc.no. Archived from the original on 17 October 2020. Retrieved 22 September 2020.
- ↑ 6.0 6.1 "WHOCC - ATC/DDD Index". www.whocc.no. Archived from the original on 1 November 2020. Retrieved 22 September 2020.
- ↑ Davanzo, R; Bua, J; Paloni, G; Facchina, G (November 2014). "Breastfeeding and migraine drugs". European Journal of Clinical Pharmacology (Review). 70 (11): 1313–24. doi:10.1007/s00228-014-1748-0. PMID 25217187.
- ↑ 8.0 8.1 8.2 Davies, NM (February 1998). "Clinical pharmacokinetics of ibuprofen: The first 30 years". Clinical Pharmacokinetics. 34 (2): 101–54. doi:10.2165/00003088-199834020-00002. PMID 9515184.
- ↑ Grosser, T; Ricciotti, E; FitzGerald, GA (August 2017). "The Cardiovascular Pharmacology of Nonsteroidal Anti-Inflammatory Drugs". Trends in Pharmacological Sciences (Review). 38 (8): 733–48. doi:10.1016/j.tips.2017.05.008. PMC 5676556. PMID 28651847.
- ↑ "Brufen Tablets And Syrup" (PDF). Therapeutic Goods Administration. 31 July 2012. Archived from the original on 20 August 2016. Retrieved 8 May 2014.
- ↑ 11.0 11.1 11.2 11.3 British National Formulary, March 2014–September 2014 (2014 ed.). London: British Medical Association. 2014. pp. 686–688. ISBN 978-0857110862.
- ↑ "Ibuprofen Pregnancy and Breastfeeding Warnings". Drugs.com. Archived from the original on 9 September 2017. Retrieved 22 May 2016.
- ↑ 13.0 13.1 13.2 13.3 13.4 13.5 Halford, GM; Lordkipanidzé, M; Watson, SP (2012). "50th anniversary of the discovery of ibuprofen: an interview with Dr Stewart Adams". Platelets. 23 (6): 415–22. doi:10.3109/09537104.2011.632032. PMID 22098129.
- ↑ "Chemistry in your cupboard | Nurofen". Archived from the original on 5 June 2014.
- ↑ World Health Organization (2019). World Health Organization model list of essential medicines: 21st list 2019. Geneva: World Health Organization. hdl:10665/325771. WHO/MVP/EMP/IAU/2019.06. License: CC BY-NC-SA 3.0 IGO.
- ↑ 16.0 16.1 "Ibuprofen". Archived from the original on 13 February 2021. Retrieved 12 January 2016.
- ↑ 17.0 17.1 "The Top 300 of 2020". ClinCalc. Archived from the original on 12 February 2021. Retrieved 11 April 2020.
- ↑ 18.0 18.1 "Ibuprofen - Drug Usage Statistics". ClinCalc. Archived from the original on 12 April 2020. Retrieved 11 April 2020.
- ↑ "10.1.1 Non-steroidal anti-inflammatory drugs". British National Formulary. Archived from the original on 17 November 2016. Retrieved 13 April 2016.
- ↑ Joint Formulary Committee (2013). British National Formulary (BNF) (65 ed.). London, UK: Pharmaceutical Press. pp. 665, 671. ISBN 978-0-85711-084-8.
- ↑ 21.0 21.1 21.2 Rossi, S, ed. (2013). Australian Medicines Handbook (2013 ed.). Adelaide: The Australian Medicines Handbook Unit Trust. ISBN 978-0-9805790-9-3.
- ↑ Alabed, S; Cabello, JB; Irving, GJ; Qintar, M; Burls, A (August 2014). "Colchicine for pericarditis" (PDF). Cochrane Database of Systematic Reviews (Review). 8 (8): CD010652. doi:10.1002/14651858.CD010652.pub2. PMID 25164988. Archived (PDF) from the original on 22 September 2017. Retrieved 2 August 2020.
- ↑ Rostas, SE; McPherson, CC (2016). "Pharmacotherapy for Patent Ductus Arteriosus: Current Options and Outstanding Questions". Current Pediatric Reviews (Review). 12 (2): 110–9. doi:10.2174/157339631202160506002028. PMID 27197952.
- ↑ Tan, Eunicia; Braithwaite, Irene; McKinlay, Christopher J. D.; Dalziel, Stuart R. (30 October 2020). "Comparison of Acetaminophen (Paracetamol) With Ibuprofen for Treatment of Fever or Pain in Children Younger Than 2 Years: A Systematic Review and Meta-analysis". JAMA Network Open. 3 (10): e2022398. doi:10.1001/jamanetworkopen.2020.22398.
- ↑ Beaver, WT (2003). "Review of the analgesic efficacy of ibuprofen". Int J Clin Pract Suppl (135): 13–7. PMID 12723741.
- ↑ Fanos, V; Antonucci, R; Zaffanello, M (2010). "Ibuprofen and acute kidney injury in the newborn". Turk. J. Pediatr. 52 (3): 231–8. PMID 20718179.
- ↑ Cite error: Invalid
<ref>tag; no text was provided for refs namedwho - ↑ 28.0 28.1 "IBUPROFEN oral - Essential drugs". medicalguidelines.msf.org. Archived from the original on 29 August 2021. Retrieved 30 August 2020.
- ↑ Castellsague J, Riera-Guardia N, Calingaert B, Varas-Lorenzo C, Fourrier-Reglat A, Nicotra F, et al. (13 December 2012). "Individual NSAIDs and Upper Gastrointestinal Complications". Drug Safety. 35 (12): 1127–1146. doi:10.1007/BF03261999. ISSN 0114-5916. PMC 3714137. PMID 23137151.
- ↑ Ayres, JG; Fleming, D; Whittington, R (9 May 1987). "Asthma death due to ibuprofen". Lancet. 1 (8541): 1082. doi:10.1016/S0140-6736(87)90499-5. PMID 2883408.
- ↑ Baselt, R (2008). Disposition of Toxic Drugs and Chemicals in Man (8th ed.). Foster City, USA: Biomedical Publications. pp. 758–761.
- ↑ Forman, JP; Stampfer, MJ; Curhan, GC (September 2005). "Non-narcotic analgesic dose and risk of incident hypertension in US women". Hypertension. 46 (3): 500–7. doi:10.1161/01.HYP.0000177437.07240.70. PMID 16103274.
- ↑ Hippisley-Cox, J; Coupland, C (11 June 2005). "Risk of myocardial infarction in patients taking cyclo-oxygenase-2 inhibitors or conventional non-steroidal anti-inflammatory drugs: population based nested case-control analysis". British Medical Journal. 330 (7504): 1366. doi:10.1136/bmj.330.7504.1366. PMC 558288. PMID 15947398.
- ↑ "FDA Drug Safety Communication: FDA strengthens warning that non-aspirin nonsteroidal anti-inflammatory drugs (NSAIDs) can cause heart attacks or strokes". U.S. Food and Drug Administration (FDA). 9 July 2015. Archived from the original on 28 October 2019. Retrieved 9 July 2015.
- Lay summary in: https://www.fda.gov/consumers/consumer-updates/fda-strengthens-warning-heart-attack-and-stroke-risk-non-steroidal-anti-inflammatory-drugs.
{{cite web}}: Missing or empty|title=(help)
- Lay summary in: https://www.fda.gov/consumers/consumer-updates/fda-strengthens-warning-heart-attack-and-stroke-risk-non-steroidal-anti-inflammatory-drugs.
- ↑ "Ibuprofen- and dexibuprofen-containing medicines". European Medicines Agency (EMA). 22 May 2015. EMA/325007/2015. Archived from the original on 28 October 2019. Retrieved 28 October 2019.
- ↑ "High-dose ibuprofen (≥2400mg/day): small increase in cardiovascular risk". Medicines and Healthcare products Regulatory Agency (MHRA). 26 June 2015. Archived from the original on 28 October 2019. Retrieved 28 October 2019.
- ↑ Chan, LS (12 June 2014). Hall, R; Vinson, RP; Nunley, JR; Gelfand, JM; Elston, DM (eds.). "Bullous Pemphigoid Clinical Presentation". Medscape Reference. United States: WebMD. Archived from the original on 10 November 2011.
- ↑ Bergner, T; Przybilla, B (January 1992). "Photosensitization caused by ibuprofen". Journal of the American Academy of Dermatology. 26 (1): 114–6. doi:10.1016/0190-9622(92)70018-b. PMID 1531054.
- ↑ Raksha, MP; Marfatia, YS (2008). "Clinical study of cutaneous drug eruptions in 200 patients". Indian J Dermatol Venereol Leprol. 74 (1): 80. doi:10.4103/0378-6323.38431. PMID 18193504.
- ↑ Ward, KE; Archambault, R; Mersfelder, TL (1 February 2010). "Severe adverse skin reactions to nonsteroidal antiinflammatory drugs: A review of the literature". American Journal of Health-System Pharmacy. 67 (3): 206–13. doi:10.2146/ajhp080603. PMID 20101062.
- ↑ 41.0 41.1 41.2 Rainsford, K.D. (2012). Ibuprofen: Pharmacology, Therapeutics and Side Effects. London: Springer.
- ↑ "Ibuprofen". Drugs.com. Archived from the original on 6 August 2011.
- ↑ "Information for Healthcare Professionals: Concomitant Use of Ibuprofen and Aspirin". U.S. Food and Drug Administration (FDA). September 2006. Archived from the original on 13 November 2010. Retrieved 22 November 2010.
- Lay summary in: "Information about Taking Ibuprofen and Aspirin Together".
- ↑ Kanabar, Dipak J. (2017). "A clinical and safety review of paracetamol and ibuprofen in children". Inflammopharmacology. 25 (1): 1–9. doi:10.1007/s10787-016-0302-3. ISSN 0925-4692. PMC 5306275. PMID 28063133.
- ↑ McElwee, NE; Veltri, JC; Bradford, DC; Rollins, DE (June 1990). "A prospective, population-based study of acute ibuprofen overdose: complications are rare and routine serum levels not warranted". Annals of Emergency Medicine. 19 (6): 657–62. doi:10.1016/S0196-0644(05)82471-0. PMID 2188537.
- ↑ Vale, JA; Meredith, TJ (January 1986). "Acute poisoning due to non-steroidal anti-inflammatory drugs. Clinical features and management". Medical Toxicology. 1 (1): 12–31. doi:10.1007/BF03259825. PMID 3537613.
- ↑ 47.0 47.1 Volans, G; Hartley, V; McCrea, S; Monaghan, J (March–April 2003). "Non-opioid analgesic poisoning". Clinical Medicine. 3 (2): 119–23. doi:10.7861/clinmedicine.3-2-119. PMC 4952728. PMID 12737366.
- ↑ Seifert, SA; Bronstein, AC; McGuire, T (2000). "Massive ibuprofen ingestion with survival". Journal of Toxicology. Clinical Toxicology. 38 (1): 55–7. doi:10.1081/clt-100100917. PMID 10696926.
- ↑ American Academy Of Clinical Toxicology (2004). "Position paper: Ipecac syrup". Journal of Toxicology. Clinical Toxicology. 42 (2): 133–143. doi:10.1081/CLT-120037421. PMID 15214617.
- ↑ Hall, AH; Smolinske, SC; Conrad, FL; Wruk, KM; Kulig, KW; Dwelle, TL; Rumack, BH (November 1986). "Ibuprofen overdose: 126 cases". Annals of Emergency Medicine. 15 (11): 1308–13. doi:10.1016/S0196-0644(86)80617-5. PMID 3777588.
- ↑ Verma, P; Clark, CA; Spitzer, KA; Laskin, CA; Ray, J; Koren, G (July 2012). "Use of non-aspirin NSAIDs during pregnancy may increase the risk of spontaneous abortion". Evidence-Based Nursing. 15 (3): 76–7. doi:10.1136/ebnurs-2011-100439. PMID 22411163.
- ↑ Daniel, S; Koren, G; Lunenfeld, E; Bilenko, N; Ratzon, R; Levy, A (March 2014). "Fetal exposure to nonsteroidal anti-inflammatory drugs and spontaneous abortions". Canadian Medical Association Journal. 186 (5): E177–82. doi:10.1503/cmaj.130605. PMC 3956584. PMID 24491470.
- ↑ "Ibuprofen Pathway, Pharmacodynamics". PharmGKB. Retrieved 10 February 2024.
- ↑ Rao, P; Knaus, EE (20 September 2008). "Evolution of nonsteroidal anti-inflammatory drugs (NSAIDs): cyclooxygenase (COX) inhibition and beyond". Journal of Pharmacy & Pharmaceutical Sciences. 11 (2): 81s–110s. doi:10.18433/J3T886. PMID 19203472.
- ↑ Kakuta, H; Zheng, X; Oda, H; Harada, S; Sugimoto, Y; Sasaki, K; Tai, A (24 April 2008). "Cyclooxygenase-1-selective inhibitors are attractive candidates for analgesics that do not cause gastric damage. design and in vitro/in vivo evaluation of a benzamide-type cyclooxygenase-1 selective inhibitor". Journal of Medicinal Chemistry. 51 (8): 2400–11. doi:10.1021/jm701191z. PMID 18363350.
- ↑ 56.0 56.1 "Ibuprofen". DrugBank. Archived from the original on 21 July 2014. Retrieved 24 July 2014.
- ↑ Bushra, R; Aslam, N (July 2010). "An overview of clinical pharmacology of Ibuprofen". Oman Medical Journal. 25 (3): 155–1661. doi:10.5001/omj.2010.49. PMC 3191627. PMID 22043330.
- ↑ 58.0 58.1 Brayfield, A, ed. (14 January 2014). "Ibuprofen". Martindale: The Complete Drug Reference. London, UK: Pharmaceutical Press. Archived from the original on 28 August 2021. Retrieved 26 June 2014.
- ↑ "The Synthesis of Ibuprofen". Royal Society of Chemistry. Archived from the original on 22 November 2018. Retrieved 22 November 2018.
- ↑ Tracy, TS; Hall, SD (March–April 1992). "Metabolic inversion of (R)-ibuprofen. Epimerization and hydrolysis of ibuprofenyl-coenzyme A.". Drug Metabolism and Disposition. 20 (2): 322–7. PMID 1352228.
- ↑ Chen, CS; Shieh, WR; Lu, PH; Harriman, S; Chen, CY (12 July 1991). "Metabolic stereoisomeric inversion of ibuprofen in mammals". Biochimica et Biophysica Acta (BBA) - Protein Structure and Molecular Enzymology. 1078 (3): 411–7. doi:10.1016/0167-4838(91)90164-U. PMID 1859831.
- ↑ Reichel, C; Brugger, R; Bang, H; Geisslinger, G; Brune, K (April 1997). "Molecular cloning and expression of a 2-arylpropionyl-coenzyme A epimerase: a key enzyme in the inversion metabolism of ibuprofen" (PDF). Molecular Pharmacology. 51 (4): 576–82. doi:10.1124/mol.51.4.576. PMID 9106621. Archived from the original (PDF) on 2 March 2019. Retrieved 2 August 2020.
- ↑ Adams, SS (April 1992). "The propionic acids: a personal perspective". Journal of Clinical Pharmacology. 32 (4): 317–23. doi:10.1002/j.1552-4604.1992.tb03842.x. PMID 1569234.
- ↑ 64.0 64.1 Rainsford, KD (April 2003). "Discovery, mechanisms of action and safety of ibuprofen". International Journal of Clinical Practice. Supplement (135): 3–8. PMID 12723739.
- ↑ Lambert, Victoria (8 October 2007). "Dr Stewart Adams: 'I tested ibuprofen on my hangover'". The Daily Telegraph. UK. Archived from the original on 18 November 2015. Retrieved 23 October 2015.
- ↑ 66.0 66.1 "Chemical landmark plaque honours scientific discovery past and future" (Press release). Royal Society of Chemistry (RSC). 21 November 2013. Archived from the original on 27 September 2018. Retrieved 2 August 2020.
- ↑ "Written submission to the NDAC meeting on risks of NSAIDs presented by the International Ibuprofen Foundation". U.S. Food and Drug Administration (FDA). August 2002. Archived from the original on 15 August 2013. Retrieved 20 March 2014.
- ↑ "New Drug Application (NDA): 017463". U.S. Food and Drug Administration (FDA). Archived from the original on 28 October 2019. Retrieved 28 October 2019.
- ↑ "New Drug Application (NDA): 018989". U.S. Food and Drug Administration (FDA). Archived from the original on 28 October 2019. Retrieved 28 October 2019.
- ↑ "Ibuprofen". U.S. Food and Drug Administration (FDA). Archived from the original on 16 September 2011.
{{cite web}}: CS1 maint: unfit URL (link) - ↑ "Drug Approval Package: Caldolor (Ibuprofen) NDA #022348". U.S. Food and Drug Administration (FDA). 11 March 2010. Archived from the original on 19 October 2012.
- ↑ "FDA Approves Injectable Form of Ibuprofen" (Press release). U.S. Food and Drug Administration (FDA). 11 June 2009. Archived from the original on 18 October 2012.
- ↑ Wong, RC; Kang, S; Heezen, JL; Voorhees, JJ; Ellis, CN (December 1984). "Oral ibuprofen and tetracycline for the treatment of acne vulgaris". Journal of the American Academy of Dermatology. 11 (6): 1076–81. doi:10.1016/S0190-9622(84)80192-9. PMID 6239884.
- ↑ "In Japan, an OTC ibuprofen ointment (Fukidia) for alleviating adult acne has been launched". Inpharma. 1 (1530): 18. 25 March 2006. doi:10.2165/00128413-200615300-00043. ISSN 1173-8324.
- ↑ Zawada ET, Jr (May 1982). "Renal consequences of nonsteroidal antiinflammatory drugs". Postgraduate Medicine. 71 (5): 223–30. doi:10.1080/00325481.1982.11716077. PMID 7041104.
- ↑ Miguel-Álvarez, M; Santos-Lozano, A; Sanchis-Gomar, F; Fiuza-Luces, C; Pareja-Galeano, H; Garatachea, N; Lucia, A (February 2015). "Non-steroidal anti-inflammatory drugs as a treatment for Alzheimer's disease: a systematic review and meta-analysis of treatment effect". Drugs & Aging. 32 (2): 139–47. doi:10.1007/s40266-015-0239-z. PMID 25644018.
- ↑ Wang, J; Tan, L; Wang, HF; Tan, CC; Meng, XF; Wang, C; Tang, SW; Yu, JT (2015). "Anti-inflammatory drugs and risk of Alzheimer's disease: an updated systematic review and meta-analysis". Journal of Alzheimer's Disease. 44 (2): 385–96. doi:10.3233/JAD-141506. PMID 25227314.
- ↑ Chen, H; Jacobs, E; Schwarzschild, MA; McCullough, ML; Calle, EE; Thun, MJ; Ascherio, A (December 2005). "Nonsteroidal antiinflammatory drug use and the risk for Parkinson's disease". Annals of Neurology. 58 (6): 963–7. doi:10.1002/ana.20682. PMID 16240369.
- ↑ Bower, JH; Ritz, B (8 March 2011). "Is the answer for Parkinson disease already in the medicine cabinet?: Unfortunately not". Neurology. 76 (10): 854–5. doi:10.1212/WNL.0b013e31820f2e7a. PMID 21368280.
- ↑ Gao, X; Chen, H; Schwarzschild, MA; Ascherio, A (8 March 2011). "Use of ibuprofen and risk of Parkinson disease". Neurology. 76 (10): 863–9. doi:10.1212/WNL.0b013e31820f2d79. PMC 3059148. PMID 21368281.
- ↑ McSharry, C (May 2011). "Parkinson disease: Could over-the-counter treatment protect against Parkinson disease?". Nature Reviews. Neurology. 7 (5): 244. doi:10.1038/nrneurol.2011.49. PMID 21555992.
- ↑ Gleason, JM; Slezak, JM; Jung, H; Reynolds, K; Van den Eeden, SK; Haque, R; Quinn, VP; Loo, RK; Jacobsen, SJ (April 2011). "Regular nonsteroidal anti-inflammatory drug use and erectile dysfunction". The Journal of Urology. 185 (4): 1388–93. doi:10.1016/j.juro.2010.11.092. PMID 21334642.
- ↑ Abebe, W. (1 December 2002). "Herbal medication: potential for adverse interactions with analgesic drugs". Journal of Clinical Pharmacy and Therapeutics. 27 (6): 391–401. doi:10.1046/j.1365-2710.2002.00444.x. ISSN 0269-4727. PMID 12472978.
External links
| External sites: | |
|---|---|
| Identifiers: |
- GB patent 971700, Stewart Sanders Adams & John Stuart Nicholson, "Anti-Inflammatory Agents", published 1964-09-30, assigned to BOOTS PURE DRUG CO LTD
- Pages using duplicate arguments in template calls
- Pages with reference errors
- CS1 errors: missing title
- CS1 errors: bare URL
- CS1 maint: unfit URL
- Articles with hatnote templates targeting a nonexistent page
- Use dmy dates from November 2014
- Articles with invalid date parameter in template
- Drugs with non-standard legal status
- Chemical articles with unknown parameter in Infobox drug
- Chemical articles without CAS registry number
- Articles without EBI source
- Chemical pages without ChemSpiderID
- Chemical pages without DrugBank identifier
- Articles without KEGG source
- Articles without UNII source
- Drugs missing an ATC code
- Drug has EMA link
- Drugboxes which contain changes to watched fields
- All articles with failed verification
- Articles with failed verification from October 2019
- Articles containing potentially dated statements from 2016
- All articles containing potentially dated statements
- Analgesics
- British inventions
- Hepatotoxins
- Johnson & Johnson brands
- Pfizer brands
- Nephrotoxins
- Nonsteroidal anti-inflammatory drugs
- Propionic acids
- Racemate
- World Health Organization essential medicines
- RTT

