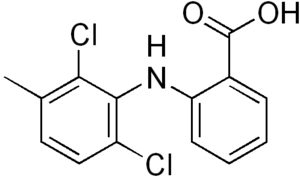
Meclofenamic acid
 | |
| Names | |
|---|---|
| Trade names | Meclomen, Ponstel, other |
| Other names | Meclofenamate, meclofenamate sodium |
| |
| Clinical data | |
| Drug class | Nonsteroidal anti-inflammatory drugs (NSAIDs)[1] |
| Main uses | Pain, inflammation[1] |
| Side effects | Headache, dizziness, nausea, abdominal pain, rash[1] |
| Routes of use | By mouth |
| Typical dose | 50 to 100 mg TID or QID[1] |
| External links | |
| AHFS/Drugs.com | Monograph |
| Chemical and physical data | |
| Formula | C14H11Cl2NO2 |
| Molar mass | 296.15 g·mol−1 |
Meclofenamic acid, also known as meclofenamate, is a nonsteroidal anti-inflammatory drugs (NSAIDs) used for pain and inflammation.[1] This includes in conditions such as painful periods, osteoarthritis, and rheumatoid arthritis.[1] It is taken by mouth.[1]
Common side effects may include headache, dizziness, nausea, abdominal pain, and rash.[1] Other side effects may include gastrointestinal bleeding, swelling, allergic reactions, stomach perforation, and stroke.[1] Use in the later part of pregnancy may harm the baby.[1] It works by blocking COX-1 and COX-2 decreasing the formation of prostaglandins.[1]
Meclofenamic acid was approved for medical use in the United States in 1980.[1] It is available as a generic medication.[2] In the United States 30 pills of 250 mg costs about 46 USD.[2] It is sold under the brand name Meclomen among others.[2]
Medical use
Dosage
It is used at a dose of 50 to 100 mg three to four times per day.[1]
Side effects
In October 2020, the U.S. Food and Drug Administration (FDA) required the drug label to be updated for all nonsteroidal anti-inflammatory medications to describe the risk of kidney problems in unborn babies that result in low amniotic fluid.[3][4] They recommend avoiding NSAIDs in pregnant women at 20 weeks or later in pregnancy.[3][4]
History
Scientists led by Claude Winder from Parke-Davis invented meclofenamate sodium in 1964, along with fellow members of the class, mefenamic acid in 1961 and flufenamic acid in 1963.[5]: 718 Patents on the drug expired in 1985[6]: 295 and several generics were introduced in the US, but as of July 2015 only Mylan still sold it.[7][8]
Other animals
Horses
Meclofenamic acid is sold under the trade name "Arquel" for use in horses, and is administered as an oral granule form at a dose of 2.2 mg/kg/day.[9] It has a relatively slow onset of action, taking 36–48 hours for full effect,[10] and is most useful for treatment of chronic musculoskeletal disease.[11] It has been found to be beneficial for the treatment of navicular syndrome, laminitis, and osteoarthritis,[10] in some cases having a more profound effect than the commonly used NSAID phenylbutazone.[12] However, due to cost, it is not routinely used in practice. Toxicity due to excessive dosage is similar to that of phenylbutazone, including depression, anorexia, weight loss, edema, diarrhea, oral ulceration, and decreased hematocrit.[12]
References
- ↑ 1.00 1.01 1.02 1.03 1.04 1.05 1.06 1.07 1.08 1.09 1.10 1.11 1.12 "Meclofenamate Monograph for Professionals". Drugs.com. Archived from the original on 25 January 2021. Retrieved 15 November 2021.
- ↑ 2.0 2.1 2.2 "Mefenamic Acid Prices, Coupons & Savings Tips - GoodRx". GoodRx. Archived from the original on 9 October 2021. Retrieved 15 November 2021.
- ↑ 3.0 3.1 "FDA Warns that Using a Type of Pain and Fever Medication in Second Half of Pregnancy Could Lead to Complications". U.S. Food and Drug Administration (FDA) (Press release). 15 October 2020. Archived from the original on 16 October 2020. Retrieved 15 October 2020.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ↑ 4.0 4.1 "NSAIDs may cause rare kidney problems in unborn babies". U.S. Food and Drug Administration. 21 July 2017. Archived from the original on 17 October 2020. Retrieved 15 October 2020.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ↑ Whitehouse M (2009). "Drugs to Treat Inflammation: A Historical Overview.". In Rahman A, et al. (eds.). Frontiers in Medicinal Chemistry. Vol. 4. Bentham Science Publishers. pp. 707–729. ISBN 978-1-60805-207-3.
- ↑ United States. Congress. Office of Technology Assessment Pharmaceutical R & D: Costs, Risks & Rewards Archived 2016-04-30 at the Wayback Machine DIANE Publishing, 1993 ISBN 978-0-7881-0468-8
- ↑ "Meclofenamate sodium ANDAs". U.S. Food and Drug Administration. Archived from the original on 4 June 2015. Retrieved 3 July 2015.
- ↑ "Mylan label for meclofenamate sodium". Daily Med. U.S. Food and Drug Administration. October 2013. Archived from the original on 4 July 2015. Retrieved 3 July 2015.
- ↑ McIlwraith CW, Frisbie DD, Kawcak CE (2001). "Nonsteroidal Anti-Inflammatory Drugs". Proc. AAEP. 47: 182–187.
- ↑ 10.0 10.1 Cotter GH, Riley WF, Beck CC, Coppock RW (1973). "Arquel (Cl- 1583). A new nonsteroidal anti-inflammatory drug for horses". Proceedings. Am Assoc Equine Practnr. 19: 81–90.
- ↑ Snow DH, Baxter P, Whiting B (June 1981). "The pharmacokinetics of meclofenamic acid in the horse". Journal of Veterinary Pharmacology and Therapeutics. 4 (2): 147–56. doi:10.1111/j.1365-2885.1981.tb00724.x. PMID 7349327.
- ↑ 12.0 12.1 Lees P, Higgins AJ (March 1985). "Clinical pharmacology and therapeutic uses of non-steroidal anti-inflammatory drugs in the horse". Equine Veterinary Journal. 17 (2): 83–96. doi:10.1111/j.2042-3306.1985.tb02056.x. PMID 3987667.
External links
| Identifiers: |
|
|---|
- Pages using duplicate arguments in template calls
- Wikipedia articles incorporating the PD-notice template
- Webarchive template wayback links
- Chemical articles with unknown parameter in Infobox drug
- Chemical articles without CAS registry number
- Articles without EBI source
- Chemical pages without ChemSpiderID
- Chemical pages without DrugBank identifier
- Articles without KEGG source
- Articles without InChI source
- Articles without UNII source
- Drugs missing an ATC code
- Drugs with no legal status
- Articles containing unverified chemical infoboxes
- Anthranilic acids
- Chloroarenes
- Equine medications
- GABAA receptor positive allosteric modulators
- RTT