Phenprobamate
 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | International Drug Names |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Elimination half-life | 5–8 hours |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.010.552 |
| Chemical and physical data | |
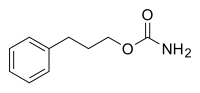
| Formula | C10H13NO2 |
| Molar mass | 179.219 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Phenprobamate (Gamaquil, Isotonil, Actozine) is a centrally acting skeletal muscle relaxant, with additional sedative and anticonvulsant effects.[2][unreliable source?] Overdose is similar to barbiturates. Its mechanism of action is probably similar to meprobamate. Phenprobamate has been used in humans as an anxiolytic, and is still sometimes used in general anesthesia and for treating muscle cramps and spasticity. Phenprobamate is still used in some European countries, but it has generally been replaced by newer drugs. Phenprobamate is metabolized by oxidative degradation of the carbamate group and ortho-hydroxylation of the benzene ring, and is eliminated in urine by the kidneys.[citation needed]
Doses range from 400 to 800 mg, up to 3 times a day.[3]
References
- ^ Anvisa (2023-03-31). "RDC Nº 784 - Listas de Substâncias Entorpecentes, Psicotrópicas, Precursoras e Outras sob Controle Especial" [Collegiate Board Resolution No. 784 - Lists of Narcotic, Psychotropic, Precursor, and Other Substances under Special Control] (in Brazilian Portuguese). Diário Oficial da União (published 2023-04-04). Archived from the original on 2023-08-03. Retrieved 2023-08-16.
- ^ Demir B, Demir Y, Aksoy I, Kilic OH, Gucyetmez V, Savas HA (June 2015). "Phenprobamate dependence: a case report". Addictive Behaviors. 45: 232–3. doi:10.1016/j.addbeh.2015.01.037. PMID 25727392.
- ^ "(meprobamate) dosing, indications, interactions, adverse effects, and more". Medscape. WebMD LLC. Retrieved 21 March 2024.
Further reading
- Tasdemir HA, Yildiran A, Islek I, Sancak R, Dilber C (May 2002). "Haemoperfusion may be useful in phenprobamate and polypharmacy intoxication of paediatric patients". Nephrology, Dialysis, Transplantation. 17 (5): 941. doi:10.1093/ndt/17.5.941. PMID 11981094.
External links
- CS1 Brazilian Portuguese-language sources (pt-br)
- Articles with short description
- Short description is different from Wikidata
- Articles with changed CASNo identifier
- ECHA InfoCard ID from Wikidata
- Chemical pages without DrugBank identifier
- Drugboxes which contain changes to verified fields
- All articles lacking reliable references
- Articles lacking reliable references from March 2024
- All articles with unsourced statements
- Articles with unsourced statements from March 2024
- Muscle relaxants
- Carbamates
- GABAA receptor positive allosteric modulators
- All stub articles
- Musculoskeletal system drug stubs