Lubiprostone
 | |
| Names | |
|---|---|
| Trade names | Amitiza, others |
| Other names | RU-0211 SPI-0211 |
| |
| Clinical data | |
| Main uses | Chronic constipation of unknown cause, irritable bowel syndrome associated constipation[1] |
| Side effects | Nausea, diarrhea, abdominal pain, swelling, tiredness[1] |
| Pregnancy category |
|
| Routes of use | By mouth |
| Typical dose | 8 to 24 mcg BID[1] |
| External links | |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a607034 |
| Legal | |
| License data |
|
| Legal status |
|
| Pharmacokinetics | |
| Bioavailability | Negligible |
| Protein binding | 94% |
| Metabolism | Extensive, CYP not involved |
| Elimination half-life | Unknown (lubiprostone) 0.9–1.4 hours (main metabolite) |
| Excretion | Kidney (60%) and fecal (30%) |
| Chemical and physical data | |
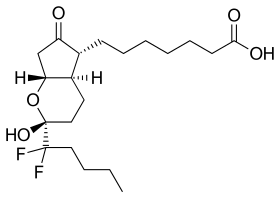
| Formula | C20H32F2O5 |
| Molar mass | 390.468 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Lubiprostone, sold under the trade name Amitiza among others, is a medication used to treat chronic constipation of unknown cause and irritable bowel syndrome associated constipation.[1] It is taken by mouth.[1]
Common side effects include nausea, diarrhea, abdominal pain, swelling, and tiredness.[1] Other side effects may include shortness of breath.[1] Safety in pregnancy is unclear.[1] It works by activating certain chloride channels in the intestines which increases fluid release.[1]
Lubiprostone was approved for medical use in the United States in 2006 and Canada in 2015.[1][2] In the United States a month of medication costs about 290 USD as of 2021.[3] A generic version was approved in 2021 in the USA.[4] It is not commercially available in the United Kingdom.[5]
Medical uses
Lubiprostone is used for the treatment of chronic constipation of unknown cause in adults, as well as irritable bowel syndrome associated with constipation in women.[6]
Lubiprostone is approved to treat chronic idiopathic constipation (CIC) in adults.
Lubiprostone is also approved to treat opioid-induced constipation, in adults with chronic non-cancer pain. The effectiveness of lubiprostone has not been established in patients who are taking a diphenylheptane opioid (e.g., methadone).
Lubiprostone is approved to treat irritable bowel syndrome with constipation (IBS-C) in women 18 years of age and older.[7]
Dosage
It is used at a dose of 8 mcg twice per day in IBS and 24 mcg twice per day in constipation of unknown cause.[1]
Side effects
Common side event include nausea (31%). Other adverse events (≥5% of patients) included diarrhea (13%), headache (13%), abdominal distension (5%), abdominal pain (5%), flatulence (6%), sinusitis (5%), vomiting (5%), and fecal incontinence (1%).
Contraindications
There is no current data on use in people with liver or kidney complications. The effects on pregnancy have not been studied in humans but testing in guinea pigs resulted in fetal loss.
Amitiza is not approved for use in children. Lubiprostone is contraindicated in patients exhibiting chronic diarrhea, bowel obstruction, or diarrhea-predominant irritable bowel syndrome.
Mechanism of action
Lubiprostone is a bicyclic fatty acid derived from prostaglandin E1 that acts by specifically activating ClC-2 chloride channels on the apical aspect of gastrointestinal epithelial cells, producing a chloride-rich fluid secretion. These secretions soften the stool, increase motility, and promote spontaneous bowel movements (SBM).
Symptoms of constipation such as pain and bloating are usually improved within one week, and SBM may occur within one day.
Pharmacokinetics
Unlike many laxative products, lubiprostone does not show signs of drug tolerance, chemical dependency, or altered serum electrolyte concentration.[8] There was no rebound effect following withdrawal of treatment, but a gradual return to pre-treatment bowel movement frequency should be expected.
Minimal distribution of the drug occurs beyond the immediate gastrointestinal tissues. Lubiprostone is rapidly metabolized by reduction/oxidation, mediated by carbonyl reductase. There is no metabolic involvement of the hepatic cytochrome P450 system. The measurable metabolite, M3, exists in very low levels in plasma and makes up less than 10% of the total administered dose.
Data indicate that metabolism occurs locally in the stomach and jejunum.
Society and culture
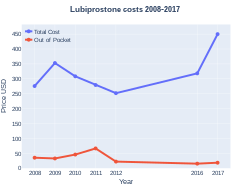
Cost
The medication is at a cost in the U.S. of $290 (USD) for 60 capsules (8 mcg)[9]
-
Lubiprostone costs (US)
-
Lubiprostone prescriptions (US)
Names
The drug is available in the United States, Japan, Switzerland, India, and Canada.
In Bangladesh and India, lubiprostone is marketed under the trade name Lubigut by Ziska Pharmaceuticals, Lubilax by Beacon Pharmaceuticals, and under the trade name Lubowel by Sun Pharmaceutical.
References
- ↑ 1.00 1.01 1.02 1.03 1.04 1.05 1.06 1.07 1.08 1.09 1.10 "Lubiprostone Monograph for Professionals". Drugs.com. Archived from the original on 22 September 2020. Retrieved 24 November 2021.
- ↑ "Health Canada New Drug Authorizations: 2015 Highlights". Health Canada. 2016-05-04. Archived from the original on 2020-02-20. Retrieved 2020-02-20.
- ↑ "Lubiprostone". Archived from the original on 31 August 2016. Retrieved 24 November 2021.
- ↑ Research, Center for Drug Evaluation and (10 February 2022). "2021 First Generic Drug Approvals". FDA. Archived from the original on 21 June 2022. Retrieved 22 October 2022.
- ↑ "Lubiprostone for treating chronic idiopathic constipation | Guidance | NICE". www.nice.org.uk. Archived from the original on 18 June 2021. Retrieved 24 November 2021.
- ↑ "Amitiza". The American Society of Health-System Pharmacists. Archived from the original on 2016-09-21. Retrieved 2018-01-23.
- ↑ "AMITIZA® (lubiprostone) 8 mcg Now Available to Treat Irritable Bowel Syndrome with Constipation in Adult Women" (Press release). Takeda Pharmaceutical Company. May 26, 2008. Archived from the original on August 29, 2021. Retrieved February 20, 2020.
- ↑ Lacy BE, Levy LC (June 2008). "Lubiprostone: a novel treatment for chronic constipation". Clinical Interventions in Aging. 3 (2): 357–64. doi:10.2147/cia.s2938. PMC 2546479. PMID 18686757.
- ↑ "Lubiprostone Prices, Coupons & Patient Assistance Programs". Drugs.com. Archived from the original on 3 March 2021. Retrieved 4 April 2021.
External links
| Identifiers: |
|
|---|
- Pages using duplicate arguments in template calls
- Chemical articles with unknown parameter in Infobox drug
- Chemical articles without CAS registry number
- Articles without EBI source
- Chemical pages without ChemSpiderID
- Chemical pages without DrugBank identifier
- Articles without KEGG source
- Articles without UNII source
- Drugs missing an ATC code
- Drugboxes which contain changes to verified fields
- Drugboxes which contain changes to watched fields
- Articles with changed EBI identifier
- Drugs acting on the gastrointestinal system and metabolism
- Fatty acids
- Laxatives
- Organofluorides
- Lactols
- Ketones
- Oxygen heterocycles
- Heterocyclic compounds (2 rings)
- Takeda Pharmaceutical Company brands
- RTT