Saxagliptin
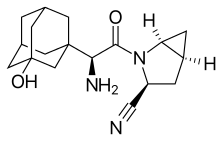 | |
| Names | |
|---|---|
| Trade names | Onglyza |
| Other names | Saxagliptin hydrochloride, BMS-477118 |
| |
| Clinical data | |
| Drug class | DPP-4 inhibitor[1] |
| Main uses | Type 2 diabetes[1] |
| Side effects | Headache, urinary tract infection, pancreatitis, joint pain, low blood sugar, heart failure[1] |
| Pregnancy category |
|
| Routes of use | By mouth (tablets) |
| Typical dose | 5 mg OD[2] |
| External links | |
| AHFS/Drugs.com | Consumer Drug Information |
| MedlinePlus | a610003 |
| Legal | |
| License data |
|
| Legal status | |
| Pharmacokinetics | |
| Bioavailability | ~75% (Tmax = 2 h) |
| Protein binding | negligible |
| Metabolism | Hepatic (CYP3A4 and CYP3A5) |
| Elimination half-life | 2.5 h (saxagliptin), 3.1 h (main metabolite) |
| Excretion | 22% (Bile), 75% (Urine) |
| Chemical and physical data | |
| Formula | C18H25N3O2 |
| Molar mass | 315.417 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Saxagliptin, sold under the brand name Onglyza, is a medication used to treat type 2 diabetes.[1] It is typically used either with metformin or when metformin is not tolerated.[2] It is taken by mouth.[1]
Common side effects include headache and urinary tract infection.[1] Other side effects may include pancreatitis, joint pain, low blood sugar, and heart failure.[1] While there is no evidence of harm in pregnancy, its use has not been well studied.[3] It is in the dipeptidyl peptidase-4 (DPP-4) inhibitor class, which indirectly increases insulin when required.[1]
Saxagliptin was approved for medical use in the United States and Europe in 2009.[4][1] In the United Kingdom 4 weeks of treatment costs the NHS about £32 as of 2021.[2] This amount in the United States costs about 400 USD.[5] It is also available in combination with other medication including metformin or dapagliflozin.[2]
Medical uses
Saxagliptin is used as monotherapy or in combination with other drugs for the treatment of type 2 diabetes. It does not appear to decrease the risk of heart attacks or strokes.[6] It increases the risk of hospitalization for heart failure by about 27%. Like other DPP-4 inhibitors, it has relatively modest HbA1c lowering ability, is associated with a relatively low risk of hypoglycemia, and does not cause weight gain.[6][7]
Saxagliptin improved mean HbA1c levels (relative to placebo) in a 24-week trial in people with type 2 diabetes.[8] Combination therapy with saxagliptin and metformin was more effective than saxagliptin or metformin monotherapy.[8] When the relative benefits of increasing the dose of a sulfonylurea or adding saxagliptin were assessed in a study of 768 patients, combination treatments were shown to have a significantly greater impact on fasting blood glucose than increasing the tested glibenclamide dose alone.[9]
Dosage
It is taken at a dose of 5 mg per day.[2]
Side effects
In those taking sulphonylureas there is an increased risk of low blood sugar.[10]
3 adverse reactions were seen higher in saxagliptin vs placebo. Table 1: Adverse Reactions (Regardless of Investigator Assessment of Causality) in Placebo-Controlled Trials* Reported in ≥ 5% of Patients Treated with ONGLYZA (saxagliptin tablets) 5 mg and More Commonly than in Patients Treated with Placebo.[11]
| ONGLYZA 5 mg N=882 | Placebo N=799 | |
|---|---|---|
| Upper respiratory tract infection | 68 (7.7) | 61 (7.6) |
| Urinary tract infection | 60 (6.8) | 49 (6.1) |
| Headache | 57 (6.5) | 47 (5.9)[11] |
- The 5 placebo-controlled trials include two monotherapy trials and one add-on combination therapy trial with each of the following: metformin, thiazolidinedione, or glyburide. Table shows 24-week data regardless of glycemic rescue.[11]
In February 2012, Bristol-Myers/Astra Zeneca distributed additional safety information on saxagliptin use in South Africa. The package insert is to be edited for South Africa. Contraindications will now include a history of sensitivity to saxagliptin (or another DPP4 inhibitor) as well as pancreatitis. Spontaneously-reported adverse events in South Africa have included anaphylaxis, angioedema and acute pancreatitis.
In a cardiovascular outcomes trial, saxagliptin treatment let to a small increase in the risk of being hospitalized for heart failure.[6] Saxagliptin may cause joint pain that can be severe and disabling.[12] Saxagliptin may increase the risk of heart failure.[13]
In April 2016, the U.S. FDA added a warning about increased risk of heart failure.[14] This was based on data in an article that concluded "DPP-4 inhibition with saxagliptin did not increase or decrease the rate of ischemic events, though the rate of hospitalization for heart failure was increased. Although saxagliptin improves glycemic control, other approaches are necessary to reduce cardiovascular risk in patients with diabetes."[6]
Tolerability
Both monotherapy and combination therapy with other agents was generally well tolerated in clinical trials.[8]
Pancreas
An association of the DPP-IV inhibitor class with pancreatic problems has been proposed, mainly based on case reports associated with the DPP-IV inhibitor sitagliptin and several incretin mimetics including exenatide. A 2013 study of the DPP-4 inhibitor sitagliptin reported found "worrisome changes in the pancreases of the rats that could lead to pancreatic cancer".[15] A second paper by the same authors reported an increase in precancerous lesions in the pancreases of organ donors who had taken GLP-1 inhibitors.[16] In response to these reports, the United States FDA and the European Medicines Agency each undertook independent reviews of all clinical and preclinical data related to the possible association of DPP-IV inhibitors with pancreatic cancer. In a joint letter to the New England Journal of Medicines, the agencies stated that "Both agencies agree that assertions concerning a causal association between incretin-based drugs and pancreatitis or pancreatic cancer, as expressed recently in the scientific literature and in the media, are inconsistent with the current data. The FDA and the EMA have not reached a final conclusion at this time regarding such a causal relationship. Although the totality of the data that have been reviewed provides reassurance, pancreatitis will continue to be considered a risk associated with these drugs until more data are available; both agencies continue to investigate this safety signal."[17]
Pharmacology
Saxagliptin is part of a class of diabetes medications called dipeptidyl peptidase-4 (DPP-4) inhibitors. DPP-4 is an enzyme that breaks down incretin hormones. As a DPP-4 inhibitor, saxagliptin slows down the breakdown of incretin hormones, increasing the level of these hormones in the body. It is this increase in incretin hormones that is responsible for the beneficial actions of saxagliptin, including increasing insulin production in response to meals and decreasing the rate of gluconeogenesis in the liver.[18]
Dipeptidyl peptidase-4's role in blood glucose regulation is thought to be through degradation of GIP[19] and the degradation of GLP-1.[19][20]
Because incretin hormones are more active in response to higher blood sugar levels (and are less active in response to low blood sugar), the risk of dangerously low blood sugar (hypoglycemia) is low with saxagliptin monotherapy.
History
Early development was solely by Bristol-Myers Squibb; in 2007 AstraZeneca joined with Bristol-Myers Squibb to co-develop the final compound and collaborate on the marketing of the drug.
Society and culture
Licensing
A New Drug Application for saxagliptin in the treatment of type 2 diabetes was submitted to the FDA in June 2008. It was based on a drug development program with 8 randomized trials: 1 phase 2 dose-ranging (2.5–100 mg/d) study; 6 phase 3, 24-week controlled trials with additional controlled follow-up from 12 to 42 months, double-blinded throughout; and one 12-week mechanism-of-action trial with a 2-year follow-up period.[21] The FDA approved saxagliptin with brand name Onglyza on July 31, 2009.[22] Saxagliptin was licensed for use throughout the European Union by the European Medicines Agency on December 1, 2009.[23] Bristol-Myers Squibb announced on 27 December 2006 that Otsuka Pharmaceutical Co. has been granted exclusive rights to develop and commercialize the compound in Japan. Under the licensing agreement, Otsuka will be responsible for all development costs, but Bristol-Myers Squibb retains rights to co-promote saxagliptin with Otsuka in Japan.[24] Further, on 11 January 2007 it was announced that Bristol-Myers Squibb and AstraZeneca would work together to complete development of the drug and in subsequent marketing.[25]
Cost
The cost in the U.S. of this medication is $487 (USD) for 30 tablets (2.5 mg)[26]
-
Saxagliptin costs (US)
-
Saxagliptin prescriptions (US)
Production
The synthesis of saxagliptin by Bristol-Myers Squibb by the amide coupling of N-Boc-3-hydroxyadamantylglycine (2) and methanoprolineamide (3) with EDC. The former is commercially available, whereas the latter is available as the N-Boc analog. The prolineamide moiety is subsequently dehydrated with trifluoroacetic anhydride to give the cyanide as the trifluoracetate ester, which is hydrolyzed. Removal of the Boc protecting group, followed by neutralization gives the desired product (1):[27]
Legal
Lawsuits have been filed in which plaintiffs who developed pancreatic cancer claim that DPP-IV inhibitors or incretins had a causative role in the development of their cancers.[28][29]
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 1.6 1.7 1.8 "SAXagliptin Monograph for Professionals". Drugs.com. Archived from the original on 23 April 2021. Retrieved 11 October 2021.
- ↑ 2.0 2.1 2.2 2.3 2.4 BNF (80 ed.). BMJ Group and the Pharmaceutical Press. September 2020 – March 2021. p. 732. ISBN 978-0-85711-369-6.
{{cite book}}: CS1 maint: date format (link) - ↑ "Saxagliptin (Onglyza) Use During Pregnancy". Drugs.com. Archived from the original on 21 April 2021. Retrieved 11 October 2021.
- ↑ "Onglyza". Archived from the original on 28 August 2021. Retrieved 11 October 2021.
- ↑ "Saxagliptin Prices, Coupons & Savings Tips - GoodRx". GoodRx. Archived from the original on 12 October 2021. Retrieved 11 October 2021.
- ↑ 6.0 6.1 6.2 6.3 Scirica, BM; Bhatt, DL; Braunwald, E; Steg, PG; Davidson, J; Hirshberg, B; Ohman, P; Frederich, R; Wiviott, SD; Hoffman, EB; Cavender, MA; Udell, JA; Desai, NR; Mosenzon, O; McGuire, DK; Ray, KK; Leiter, LA; Raz, I (Oct 3, 2013). "Saxagliptin and Cardiovascular Outcomes in Patients with Type 2 Diabetes Mellitus" (PDF). The New England Journal of Medicine. 369 (14): 1317–1326. doi:10.1056/nejmoa1307684. hdl:10044/1/40070. PMID 23992601. Archived (PDF) from the original on August 29, 2021. Retrieved June 26, 2019.
- ↑ Ali S; Fonseca V (January 2013). "Saxagliptin overview: special focus on safety and adverse effects". Expert Opin Drug Saf. 12 (1): 103–9. doi:10.1517/14740338.2013.741584. PMID 23137182. S2CID 12634714.
- ↑ 8.0 8.1 8.2 Dhillon, S; Weber, J. (2009). "Saxagliptin". Drugs. 69 (15): 2103–2114. doi:10.2165/11201170-000000000-00000. PMID 19791828. Archived from the original on 2011-10-08. Retrieved 2009-11-04.
- ↑ "New Drugs: Saxagliptin". Australian Prescriber (34): 89–91. June 2011. Archived from the original on 2014-02-02. Retrieved 2011-06-17.
- ↑ Salvo, Francesco; Moore, Nicholas; Arnaud, Mickael; Robinson, Philip; Raschi, Emanuel; De Ponti, Fabrizio; Bégaud, Bernard; Pariente, Antoine (3 May 2016). "Addition of dipeptidyl peptidase-4 inhibitors to sulphonylureas and risk of hypoglycaemia: systematic review and meta-analysis". BMJ. 353: i2231. doi:10.1136/bmj.i2231. PMC 4854021. PMID 27142267.
- ↑ 11.0 11.1 11.2 "Onglyza". RxList. Archived from the original on 2012-01-09. Retrieved 2012-01-31.
- ↑ "DPP-4 Inhibitors for Type 2 Diabetes: Drug Safety Communication - May Cause Severe Joint Pain". U.S. Food and Drug Administration (FDA). 2015-08-28. Archived from the original on 2019-12-13. Retrieved 1 September 2015.
- ↑ "FDA Drug Safety Communication: FDA adds warnings about heart failure risk to labels of type 2 diabetes medicines containing saxagliptin and alogliptin". U.S. Food and Drug Administration (FDA). 12 January 2017. Archived from the original on 11 August 2020. Retrieved 11 August 2020.
- ↑ "Safety Alerts for Human Medical Products - Diabetes Medications Containing Saxagliptin and Alogliptin: Drug Safety Communication - Risk of Heart Failure". www.fda.gov. Archived from the original on 7 April 2016. Retrieved 7 April 2016.
- ↑ Matveyenko AV, Dry S, Cox HI, et al. (July 2009). "Beneficial endocrine but adverse exocrine effects of sitagliptin in the human islet amyloid polypeptide transgenic rat model of type 2 diabetes: interactions with metformin". Diabetes. 58 (7): 1604–15. doi:10.2337/db09-0058. PMC 2699878. PMID 19403868.
- ↑ Butler AE, Campbell-Thompson M, Gurlo T, Dawson DW, Atkinson M, Butler PC (July 2013). "Marked expansion of exocrine and endocrine pancreas with incretin therapy in humans with increased exocrine pancreas dysplasia and the potential for glucagon-producing neuroendocrine tumors". Diabetes. 62 (7): 2595–604. doi:10.2337/db12-1686. PMC 3712065. PMID 23524641.
- ↑ Egan, Amy G.; Blind, Eberhard; Dunder, Kristina; De Graeff, Pieter A.; Hummer, B. Timothy; Bourcier, Todd; Rosebraugh, Curtis (2014). "Pancreatic Safety of Incretin-Based Drugs — FDA and EMA Assessment — NEJM" (PDF). New England Journal of Medicine. 370 (9): 794–7. doi:10.1056/NEJMp1314078. PMID 24571751. Archived (PDF) from the original on 2018-07-22. Retrieved 2019-09-21.
- ↑ [1] Archived 2011-08-10 at the Wayback Machine Diabetes info
- ↑ 19.0 19.1 Mentlein, R; Gallwitz, B; Schmidt, WE (15 June 1993). "Dipeptidyl-peptidase IV hydrolyses gastric inhibitory polypeptide, glucagon-like peptide-1(7-36)amide, peptide histidine methionine and is responsible for their degradation in human serum". European Journal of Biochemistry. 214 (3): 829–835. doi:10.1111/j.1432-1033.1993.tb17986.x. PMID 8100523.
- ↑ Ahrén, Bo; Landin-Olsson, Mona; Jansson, Per-Anders; Svensson, Maria; Holmes, David; Schweizer, Anja (May 2004). "Inhibition of dipeptidyl peptidase-4 reduces glycemia, sustains insulin levels, and reduces glucagon levels in type 2 diabetes". Journal of Clinical Endocrinology & Metabolism. 89 (5): 2078–2084. doi:10.1210/jc.2003-031907. PMID 15126524. Archived from the original on 2011-05-16. Retrieved 2007-01-11.
- ↑ Robert Frederich, MD; et al. (May 2010). "A Systematic Assessment of Cardiovascular Outcomes in the Saxagliptin Drug Development Program for Type 2 Diabetes". Postgraduate Medicine. 122 (3): 16–27. doi:10.3810/pgm.2010.05.2138. PMID 20463410. S2CID 10975424. Archived from the original on 2011-01-05. Retrieved 2010-07-06.
- ↑ Telegram (2 August 2009). "FDA approves diabetes drug from two area manufacturers". Worcester Telegram & Gazette Corp. Archived from the original on 2009-08-07. Retrieved 2009-08-02.
- ↑ EMA publication | www.ema.europa.eu/docs/en_GB/document.../WC500044316.pdf
- ↑ "Bristol-Myers Squibb and Otsuka Pharmaceutical Co., Ltd. Announce Exclusive Licensing Agreement for Diabetes Compound Saxagliptin in Japan" (Press release). Bristol-Myers Squibb. December 27, 2006. Archived from the original on 2021-09-25. Retrieved 2006-12-27.
- ↑ "AstraZeneca teams with Bristol-Myers on diabetes drugs". Delaware News-Journal. Associated Press. 11 January 2007. Archived from the original on September 30, 2007. Retrieved 2007-01-11.
- ↑ "Onglyza Prices, Coupons & Patient Assistance Programs". Drugs.com. Archived from the original on 22 January 2021. Retrieved 7 April 2021.
- ↑ Scott AS, Gregory SJ, Sergei K, Shelly AR, Truc V, Robert EW (2009). "Preparation of Saxagliptin, a Novel DPP-IV Inhibitor". Org. Process Res. Dev. 13 (6): 1169–1176. doi:10.1021/op900226j.
- ↑ "Latest Januvia Lawsuits Alleging Pancreatic Cancer Help: Resource4thePeople Reports Cases Continue To Be Filed in Federal Multidistrict Litigation". DG. DigitalJournal.com. October 14, 2013. Archived from the original on 2014-12-31. Retrieved 2013-10-14.
- ↑ "IN RE: INCRETIN MIMETICS PRODUCTS LIABILITY LITIGATION" (PDF). USJP. United States Judicial Panel on Multidistric Litigation. August 26, 2013. Archived (PDF) from the original on 2013-11-07. Retrieved 2013-08-26.
External links
| Identifiers: |
|
|---|
- "Saxagliptin". Drug Information Portal. U.S. National Library of Medicine. Archived from the original on 2020-07-18. Retrieved 2020-07-14.
- Banting and Best Diabetes Centre at UT dpp4
- Pages using duplicate arguments in template calls
- CS1 maint: date format
- Webarchive template wayback links
- Chemical articles with unknown parameter in Infobox drug
- Chemical articles without CAS registry number
- Articles without EBI source
- Chemical pages without ChemSpiderID
- Chemical pages without DrugBank identifier
- Articles without KEGG source
- Articles without UNII source
- Drugs missing an ATC code
- Drug has EMA link
- Drugboxes which contain changes to verified fields
- Drugboxes which contain changes to watched fields
- Articles with hatnote templates targeting a nonexistent page
- Articles with changed CASNo identifier
- Dipeptidyl peptidase-4 inhibitors
- Nitriles
- Bristol-Myers Squibb
- AstraZeneca brands
- Adamantanes
- Tertiary alcohols
- Carboxamides
- Nitrogen heterocycles
- RTT


