Alogliptin
 | |
| Names | |
|---|---|
| Trade names | Nesina, Vipidia Kazano, Vipidomet (with metformin) Oseni, Incresync (with pioglitazone) |
| Other names | Alogliptin benzoate, SYR-322 |
| |
| Clinical data | |
| Drug class | DPP-4 inhibitor (gliptin)[1] |
| Main uses | Type 2 diabetes[1] |
| Side effects | Runny nose, headache, upper respiratory tract infection[1] |
| Pregnancy category |
|
| Routes of use | By mouth |
| Typical dose | 25 mg OD[2] |
| External links | |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a613026 |
| Legal | |
| License data |
|
| Legal status | |
| Pharmacokinetics | |
| Bioavailability | 100% |
| Protein binding | 20% |
| Metabolism | Limited, liver (CYP2D6- and 3A4-mediated) |
| Elimination half-life | 12–21 hours |
| Excretion | Kidney (major) and fecal (minor) |
| Chemical and physical data | |
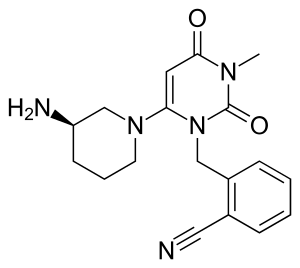
| Formula | C18H21N5O2 |
| Molar mass | 339.399 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Alogliptin, sold under the brand names Nesina and Vipidia among others, is a medication used to treat type 2 diabetes.[1] It is a second line treatment used together with diet and exercise.[3] Effects on the risk of heart disease are unclear.[1] It is taken by mouth.[2]
Common side effects include runny nose, headache, and upper respiratory tract infection.[1] Other side effects may include pancreatitis, heart failure, anaphylaxis, liver problems, and low blood sugar.[1] Safety in pregnancy is unclear.[1] It is a DPP-4 inhibitor (gliptin), which works by increasing insulin secretion from the pancreas.[1][2]
Alogliptin was approved for medical use in the United States and Europe in 2013.[1][4] In the United Kingdom 4 weeks costs the NHS about £27 as of 2021.[2] This amount in the United States is about 90 USD.[5] It is also available as a combination medication with metformin or pioglitazone.[3]
Medical uses
Alogliptin is a dipeptidyl peptidase-4 inhibitor that decreases blood sugar similar to the other.[6]
Like other members of the gliptin class, it causes little or no weight gain, exhibits relatively little risk of hypoglycemia, and has relatively modest glucose-lowering activity. Alogliptin and other gliptins are commonly used in combination with metformin in people whose diabetes cannot adequately be controlled with metformin alone.[7]
Dosage
It is taken at a dose of 25 mg once per day.[2]
Side effects
Side effects may include mild hypoglycemia based on clinical studies.[8][9][10] Alogliptin is not associated with increased weight, increased risk of cardiovascular events.[11][12] It may also cause joint pain that can be severe and disabling.[13] In April 2016, the U.S. Food and Drug Administration (FDA) added a warning about increased risk of heart failure.[14]
History
It was developed by Syrrx, a company which was acquired by Takeda Pharmaceutical Company in 2005.[15]
Market access

In December 2007, Takeda submitted a New Drug Application (NDA) for alogliptin to the United States Food and Drug Administration (USFDA),[16] after positive results from Phase III clinical trials.[17] In September 2008, the company also filed for approval in Japan,[18] winning approval in April 2010.[16] The company also filed a Marketing Authorization Application (MAA) elsewhere outside the United States, which was withdrawn in June 2009 needing more data.[18] The first USFDA NDA failed to gain approval and was followed by a pair of NDAs (one for alogliptin and a second for a combination of alogliptin and pioglitazone) in July 2011.[16] In 2012, Takeda received a negative response from the USFDA on both of these NDAs, citing a need for additional data.[16]
In 2013, the FDA approved the drug in three formulations: as a stand-alone with the brand-name Nesina, combined with metformin using the name Kazano, and when combined with pioglitazone as Oseni.
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 1.6 1.7 1.8 1.9 "DailyMed - ALOGLIPTIN tablet, film coated". dailymed.nlm.nih.gov. Archived from the original on 10 November 2021. Retrieved 14 January 2022.
- ↑ 2.0 2.1 2.2 2.3 2.4 BNF 81: March-September 2021. BMJ Group and the Pharmaceutical Press. 2021. p. 734. ISBN 978-0857114105.
- ↑ 3.0 3.1 "Alogliptin Monograph for Professionals". Drugs.com. Archived from the original on 28 January 2021. Retrieved 14 January 2022.
- ↑ "Vipidia". Archived from the original on 16 November 2021. Retrieved 14 January 2022.
- ↑ "Alogliptin Prices, Coupons & Savings Tips - GoodRx". GoodRx. Retrieved 14 January 2022.
- ↑ Saisho, Y (2015). "Alogliptin benzoate for management of type 2 diabetes". Vascular Health and Risk Management. 11: 229–43. doi:10.2147/VHRM.S68564. PMC 4401208. PMID 25914541.
- ↑ "www.aace.com" (PDF). Archived from the original (PDF) on 2018-11-01.
- ↑ Seino, Yutaka; Fujita, Tetsuya; Hiroi, Shinzo; Hirayama, Masashi; Kaku, Kohei (September 2011), "Efficacy and safety of alogliptin in Japanese patients with type 2 diabetes mellitus: a randomized, double-blind, dose-ranging comparison with placebo, followed by a long-term extension study (abstract only)", Current Medical Research and Opinion, 27 (9): 1781–1792, doi:10.1185/03007995.2011.599371, PMID 21806314, S2CID 24082863
- ↑ Kutoh, Eiji; Ukai, Yasuhiro (2012), "Alogliptin as an initial therapy in patients with newly diagnosed, drug naïve type 2 diabetes: a randomized, control trial (abstract only)", Endocrine (published January 17, 2012), 41 (3): 435–41, doi:10.1007/s12020-012-9596-0, PMID 22249941, S2CID 45948727
- ↑ Bosi, Emanuele; Ellis, G.C.; Wilson, C.A.; Fleck, P.R. (October 2011), "Alogliptin as a third oral antidiabetic drug in patients with type 2 diabetes and inadequate glycaemic control on metformin and pioglitazone: a 52-week, randomized, double-blind, active-controlled, parallel-group study", Diabetes, Obesity and Metabolism (published October 27, 2011), 13 (12): 1088–1096, doi:10.1111/j.1463-1326.2011.01463.x, PMID 21733058, S2CID 1092260
- ↑ White WB, Cannon CP, Heller SR, et al. (October 2013). "Alogliptin after acute coronary syndrome in patients with type 2 diabetes" (PDF). N. Engl. J. Med. 369 (14): 1327–35. doi:10.1056/NEJMoa1305889. hdl:10447/94479. PMID 23992602. Archived (PDF) from the original on 2021-11-05. Retrieved 2021-05-07.
- ↑ White WB, Zannad F (January 2014). "Saxagliptin, alogliptin, and cardiovascular outcomes". N. Engl. J. Med. 370 (5): 483–484. doi:10.1056/NEJMc1313880. PMID 24482824.
- ↑ "DPP-4 Inhibitors for Type 2 Diabetes: Drug Safety Communication - May Cause Severe Joint Pain". U.S. Food and Drug Administration (FDA). 2015-08-28. Archived from the original on 2019-12-13. Retrieved 1 September 2015.
- ↑ "FDA Drug Safety Communication: FDA adds warnings about heart failure risk to labels of type 2 diabetes medicines containing saxagliptin and alogliptin". U.S. Food and Drug Administration (FDA). Archived from the original on 11 August 2020. Retrieved 16 March 2018.
- ↑ "Archive copy". Archived from the original on 2014-03-08. Retrieved 2021-05-07.
{{cite web}}: CS1 maint: archived copy as title (link) - ↑ 16.0 16.1 16.2 16.3 Grogan, Kevin (April 26, 2012), "FDA wants yet more data on Takeda diabetes drug alogliptin", PharmaTimes, PharmaTimes, PharmaTimes online, archived from the original on September 24, 2015, retrieved April 26, 2012
- ↑ "Takeda Submits New Drug Application for Alogliptin (SYR-322) in the U.S." (Press release). Takeda Pharmaceutical Company. January 3, 2008. Archived from the original on December 3, 2020. Retrieved March 11, 2021.
- ↑ 18.0 18.1 "GEN News Highlights: Takeda Pulls MAA for Type 2 Diabetes Therapy". Genetic Engineering & Biotechnology News. June 4, 2009.
External links
| Identifiers: |
|
|---|
- "Alogliptin". Drug Information Portal. U.S. National Library of Medicine. Archived from the original on 2021-11-05. Retrieved 2021-05-07.
- Pages using duplicate arguments in template calls
- CS1 maint: archived copy as title
- Drugs with non-standard legal status
- Chemical articles with unknown parameter in Infobox drug
- Chemical articles without CAS registry number
- Articles without EBI source
- Chemical pages without ChemSpiderID
- Chemical pages without DrugBank identifier
- Articles without KEGG source
- Articles without UNII source
- Chemicals using indexlabels
- Drugs missing an ATC code
- Drug has EMA link
- Drugboxes which contain changes to verified fields
- Drugboxes which contain changes to watched fields
- Dipeptidyl peptidase-4 inhibitors
- Nitriles
- Piperidines
- Ureas
- Imides
- Pyrimidinediones
- Enantiopure drugs
- Takeda Pharmaceutical Company brands
- Sanofi
- RTT