Dapagliflozin
 | |||
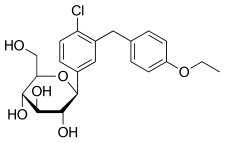 Haworth projection (bottom) | |||
| |||
| Names | |||
|---|---|---|---|
| Pronunciation | /ˌdæpəɡlɪˈfloʊzɪn/ DAP-ə-glif-LOH-zin | ||
| Trade names | Forxiga, Farxiga, Edistride, others | ||
| Other names | BMS-512148; (1S)-1,5-anhydro-1-C-{4-chloro-3-[(4-ethoxyphenyl)methyl]phenyl}-D-glucitol | ||
| |||
| Clinical data | |||
| Drug class | Sodium-glucose co-transporter 2 (SGLT2) inhibitor | ||
| Pregnancy category | |||
| Routes of use | By mouth (tablets) | ||
| External links | |||
| AHFS/Drugs.com | Monograph | ||
| Legal | |||
| License data |
| ||
| Legal status | |||
| Pharmacokinetics | |||
| Bioavailability | 78% (after 10 mg dose) | ||
| Protein binding | ~91% | ||
| Metabolism | UGT1A9 (major), CYP (minor) | ||
| Metabolites | Dapagliflozin 3-O-glucuronide (inactive) | ||
| Elimination half-life | ~12.9 hours | ||
| Excretion | Urine (75%), feces (21%)[2] | ||
| Chemical and physical data | |||
| Formula | C21H25ClO6 | ||
| Molar mass | 408.88 g·mol−1 | ||
| 3D model (JSmol) | |||
| |||
| |||
Dapagliflozin, sold under the brand name Farxiga among others, is a medication used to treat type 2 diabetes.[2] It is also used in heart failure with reduced ejection fraction.[3] In type 1 diabetes, it may be used together with insulin.[4] It is taken by mouth once a day.[2]
Common side effect include urinary tract infections, fungal infections of the groin, increased urination, nausea, and constipation.[2] Other side effects may include low blood sugar, especially when used with other diabetic medications, low blood pressure, Fournier gangrene, allergic reactions, and diabetic ketoacidosis.[2] Use is not recommended in pregnancy or breastfeeding.[4] It is of the gliflozin (SGLT2 inhibitor) class.[2]
Dapagliflozin was approved for medical use in the United States in 2014.[2] It was originally brought to market by AstraZeneca.[2] It is on the World Health Organization's List of Essential Medicines as an alternative to empagliflozin.[5] In the United States it costs about 500 USD per month as of 2020.[6] This amount costs the NHS in the United Kingdom about 40 pounds.[4] In 2017, it was the 259th most commonly prescribed medication in the United States, with more than one million prescriptions.[7][8]
Medical uses
Dapagliflozin is used along with diet and exercise to improve glycemic control in adults with type 2 diabetes and to reduce the risk of hospitalization for heart failure among adults with type 2 diabetes and known cardiovascular disease or other risk factors.[9][3] It appears less useful than empagliflozin.[10]
In the US, it is also indicated for the treatment of adults with heart failure with reduced ejection fraction to reduce the risk of cardiovascular death and hospitalization for heart failure.[3]
In the European Union it is indicated in adults for the treatment of insufficiently controlled type 2 diabetes mellitus as an adjunct to diet and exercise
- as monotherapy when metformin is considered inappropriate due to intolerance.[11]
- in addition to other medicinal products for the treatment of type 2 diabetes.[11]
and for the treatment of insufficiently controlled type 1 diabetes mellitus as an adjunct to insulin in patients with BMI ≥ 27 kg/m2, when insulin alone does not provide adequate glycaemic control despite optimal insulin therapy.[11]
One study found that it had no benefit on heart disease risk or overall risk of death in people with diabetes.[12] Another study found that in heart failure with a reduced ejection fraction, dapagliflozin reduced the risk of worsening of heart failure or progression to death from cardiovascular causes, irrespective of diabetic status.[13]
Dosage
The typical dose is 5 to 10 mg per day.[4] Generally it is used with other diabetic medications.[4]
Side effects
Since dapagliflozin leads to lots of sugar in the urine (sometimes up to about 70 grams per day) it can lead to weight loss and tiredness. The glucose acts as an osmotic diuretic (this effect is the cause of increased urine volumes in diabetes) which can lead to dehydration. The increased amount of glucose in the urine can also worsen the infections already associated with diabetes, particularly urinary tract infections and thrush (candidiasis). Rarely, use of a SGLT2 drug, including dapagliflozin, is associated with necrotizing fasciitis of the perineum, also called Fournier gangrene.[14]
Dapagliflozin is also associated with hypotensive reactions. There are concerns it may increase the risk of diabetic ketoacidosis.[15]
Dapagliflozin can cause dehydration, serious urinary tract infections and genital yeast infections.[3] Elderly people, people with kidney problems, those with low blood pressure, and people on diuretics should be assessed for their volume status and kidney function.[3] People with signs and symptoms of metabolic acidosis or ketoacidosis (acid buildup in the blood) should also be assessed.[3] Dapagliflozin can cause serious cases of necrotizing fasciitis of the perineum (Fournier's Gangrene) in people with diabetes and low blood sugar when combined with insulin.[3]
To lessen the risk of developing ketoacidosis (a serious condition in which the body produces high levels of blood acids called ketones) after surgery, the FDA has approved changes to the prescribing information for SGLT2 inhibitor diabetes medicines to recommend they be stopped temporarily before scheduled surgery. Canagliflozin, dapagliflozin, and empagliflozin should each be stopped at least three days before, and ertugliflozin should be stopped at least four days before scheduled surgery.[16]
Symptoms of ketoacidosis include nausea, vomiting, abdominal pain, tiredness, and trouble breathing.[16]
Mechanism of action
Dapagliflozin inhibits subtype 2 of the sodium-glucose transport proteins (SGLT2) which are responsible for at least 90% of the glucose reabsorption in the kidney. Blocking this transporter mechanism causes blood glucose to be eliminated through the urine.[17] In clinical trials, dapagliflozin lowered HbA1c by 0.6 versus placebo percentage points when added to metformin.[18]
Regarding its protective effects in heart failure, this is attributed primarily to haemodynamic effects, where SGLT2 inhibitors potently reduce intravascular volume through osmotic diuresis and natriuresis. This consequently may lead to a reduction in preload and afterload, thereby alleviating cardiac workload and improving left ventricular function.[19]
Selectivity
The IC50 for SGLT2 is less than one thousandth of the IC50 for SGLT1 (1.1 versus 1390 nmol/L), so that the drug does not interfere with intestinal glucose absorption.[20]
Names
Dapagliflozin is the International nonproprietary name (INN),[21] and the United States Adopted Name (USAN).[22]
There is a fixed-dose combination product dapagliflozin/metformin extended-release, called Xigduo XR.[23][24][25]
In July 2016, the fixed-dose combination of saxagliptin and dapagliflozin was approved for medical use in the European Union and is sold under the brand name Qtern.[26] The combination drug was approved for medical use in the United States in February 2017, where it is sold under the brand name Qtern.[27][28]
In May 2019, the fixed-dose combination of dapagliflozin, saxagliptin, and metformin hydrochloride as extended-release tablets was approved in the United States to improve glycemic control in adults with type 2 diabetes when used in combination with diet and exercise. The FDA granted the approval of Qternmet XR to AstraZeneca.[29] The combination drug was approved for use in the European Union in November 2019, and is sold under the brand name Qtrilmet.[30]
History
In 2012, the Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency (EMA) issued a positive opinion on the drug.[11]
Dapagliflozin was found effective in several studies in participants with type 2 and type 1 diabetes.[11] The main measure of effectiveness was the level of glycosylated haemoglobin (HbA1c), which gives an indication of how well blood glucose is controlled.[11]
In two studies involving 840 participants with type 2 diabetes, dapagliflozin when used alone decreased HbA1c levels by 0.66 percentage points more than placebo (a dummy treatment) after 24 weeks.[11] In four other studies involving 2,370 participants, adding dapagliflozin to other diabetes medicines decreased HbA1c levels by 0.54-0.68 percentage points more than adding placebo after 24 weeks.[11]
In a study involving 814 participants with type 2 diabetes, dapagliflozin used in combination with metformin was at least as effective as a sulphonylurea (another type of diabetes medicines) used with metformin.[11] Both combinations reduced HbA1c levels by 0.52 percentage points after 52 weeks.[11]
A long-term study, involving over 17,000 participants with type 2 diabetes, looked at the effects of dapagliflozin on cardiovascular (heart and circulation) disease.[11] The study indicated that dapagliflozin's effects were in line with those of other diabetes medicines that also work by blocking SGLT2.[11]
In two studies involving 1,648 participants with type 1 diabetes whose blood sugar was not controlled well enough on insulin alone, adding dapagliflozin 5 mg decreased HbA1c levels after 24 hours by 0.37% and by 0.42% more than adding placebo.[11]
Dapagliflozin was approved for medical use in the European Union in November 2012.[11] It is marketed in a number of European countries.[31]
Dapagliflozin was approved for medical use in the United States in January 2014.[32]
In 2020, the U.S. Food and Drug Administration (FDA) expanded the indications for dapagliflozin to include treatment for adults with heart failure with reduced ejection fraction to reduce the risk of cardiovascular death and hospitalization for heart failure.[3] It is the first in this particular drug class, sodium-glucose co-transporter 2 (SGLT2) inhibitors, to be approved to treat adults with New York Heart Association's functional class II-IV heart failure with reduced ejection fraction.[3]
Dapagliflozin was shown to improve survival and reduce the need for hospitalization in adults with heart failure with reduced ejection fraction.[3] The safety and effectiveness of dapagliflozin were evaluated in a randomized, double-blind, placebo-controlled study of 4,744 participants.[3] The average age of participants was 66 years and more participants were male (77%) than female.[3] To determine the drug's effectiveness, investigators examined the occurrence of cardiovascular death, hospitalization for heart failure, and urgent heart failure visits.[3] Participants were randomly assigned to receive a once-daily dose of either 10 milligrams of dapagliflozin or a placebo (inactive treatment).[3] After about 18 months, people who received dapagliflozin had fewer cardiovascular deaths, hospitalizations for heart failure, and urgent heart failure visits than those receiving the placebo.[3]
In July 2020, the FDA granted AstraZeneca a Fast Track Designation in the US for the development of dapagliflozin to reduce the risk of hospitalisation for heart failure or cardiovascular death in adults following a heart attack.[33]
In August 2020, it was reported that detailed results from the Phase III DAPA-CKD trial showed that AstraZeneca’s FARXIGA® (dapagliflozin) on top of standard of care reduced the composite measure of worsening of renal function or risk of cardiovascular (CV) or renal death by 39% compared to placebo (p<0.0001) in patients with chronic kidney disease (CKD) Stages 2-4 and elevated urinary albumin excretion. The results were consistent in patients both with and without type 2 diabetes (T2D)[34]
Society and culture
Cost
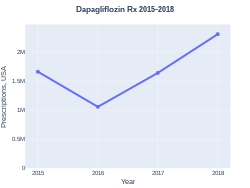
In the United States it costs about 500 USD per month as of 2020.[6] This amount costs the NHS in the United Kingdom about 40 pounds.[4] In 2017, it was the 259th most commonly prescribed medication in the United States, with more than one million prescriptions.[7][8]
-
Dapagliflozin costs (US)
-
Dapagliflozin prescriptions (US)
References
- ↑ 1.0 1.1 "Dapagliflozin (Farxiga) Use During Pregnancy". Drugs.com. 30 August 2018. Archived from the original on 17 April 2021. Retrieved 5 May 2020.
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 2.6 2.7 "Dapagliflozin Propanediol Monograph for Professionals". Drugs.com. Archived from the original on 17 February 2020. Retrieved 3 December 2020.
- ↑ 3.00 3.01 3.02 3.03 3.04 3.05 3.06 3.07 3.08 3.09 3.10 3.11 3.12 3.13 3.14 "FDA approves new treatment for a type of heart failure". U.S. Food and Drug Administration (FDA) (Press release). 5 May 2020. Archived from the original on 6 May 2020. Retrieved 5 May 2020.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ↑ 4.0 4.1 4.2 4.3 4.4 4.5 BNF 79. London: Pharmaceutical Press. March 2020. p. 725. ISBN 978-0857113658.
- ↑ World Health Organization (2023). The selection and use of essential medicines 2023: web annex A: World Health Organization model list of essential medicines: 23rd list (2023). Geneva: World Health Organization. hdl:10665/371090. WHO/MHP/HPS/EML/2023.02.
- ↑ 6.0 6.1 "Dapagliflozin Prices, Coupons & Savings Tips". GoodRx. Archived from the original on 14 June 2016. Retrieved 3 December 2020.
- ↑ 7.0 7.1 "The Top 300 of 2020". ClinCalc. Archived from the original on 12 February 2021. Retrieved 11 April 2020.
- ↑ 8.0 8.1 "Dapagliflozin - Drug Usage Statistics". ClinCalc. Archived from the original on 13 April 2020. Retrieved 11 April 2020.
- ↑ "FDA Approves Farxiga to Treat Type 2 Diabetes" (Press release). U.S. Food and Drug Administration (FDA). 8 January 2014. Archived from the original on 9 January 2014. Retrieved 15 November 2016.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ↑ Zelniker TA, Wiviott SD, Raz I, et al. (January 2019). "SGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials". Lancet. 393 (10166): 31–9. doi:10.1016/S0140-6736(18)32590-X. PMID 30424892. S2CID 53277899.
However, in patients with atherosclerotic cardiovascular disease, the effect of empagliflozin on cardiovascular death was more pro-nounced than that of canagliflozin or dapagliflozin
- ↑ 11.00 11.01 11.02 11.03 11.04 11.05 11.06 11.07 11.08 11.09 11.10 11.11 11.12 11.13 "Forxiga EPAR". European Medicines Agency (EMA). Archived from the original on 17 February 2020. Retrieved 17 February 2020. Text was copied from this source which is © European Medicines Agency. Reproduction is authorized provided the source is acknowledged.
- ↑ "Type 2 diabetes. Cardiovascular assessment of dapagliflozin: no advance". Prescrire International. 29 (211): 23. January 2020. Archived from the original on 17 February 2020. Retrieved 2 February 2020.

- ↑ McMurray JJ, Solomon SD, Inzucchi SE, et al. (November 2019). "Dapagliflozin in Patients with Heart Failure and Reduced Ejection Fraction". New England Journal of Medicine. 381 (21): 1995–2008. doi:10.1056/NEJMoa1911303. PMID 31535829.
- ↑ "FDA warns about rare occurrences of a serious infection of the genital area with SGLT2 inhibitors for diabetes". U.S. Food and Drug Administration (FDA). 9 February 2019. Archived from the original on 13 December 2019. Retrieved 16 December 2019.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ↑ "SGLT2 inhibitors: Drug Safety Communication - FDA Warns Medicines May Result in a Serious Condition of Too Much Acid in the Blood". U.S. Food and Drug Administration (FDA). 15 May 2015. Archived from the original on 27 October 2016. Retrieved 15 November 2016.
- ↑ 16.0 16.1 "FDA revises labels of SGLT2 inhibitors for diabetes to include warning". U.S. Food and Drug Administration. 19 March 2020. Archived from the original on 7 June 2020. Retrieved 6 June 2020.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ↑ "Life Sciences - Clarivate". Clarivate. Archived from the original on 2007-11-05.
- ↑ "UEndocrine: Internet Endocrinology Community". uendocrine.com. Archived from the original on 2013-02-05.
- ↑ Lan NS, Fegan PG, Yeap BB, Dwivedi G (October 2019). "The effects of sodium-glucose cotransporter 2 inhibitors on left ventricular function: current evidence and future directions". ESC Heart Fail. 6 (5): 927–935. doi:10.1002/ehf2.12505. PMC 6816235. PMID 31400090.
- ↑ Schubert-Zsilavecz, M, Wurglics, M, Neue Arzneimittel 2008/2009
- ↑ "International Nonproprietary Names for Pharmaceutical Substances (INN). Recommended International Nonproprietary Names: List 59" (PDF). World Health Organization. 2008. p. 50. Archived (PDF) from the original on 18 May 2016. Retrieved 15 November 2016.
- ↑ "Statement on a Nonproprietary Name Adopted by the USAN Council" (PDF). American Medical Association. Archived from the original (PDF) on 7 February 2012. Retrieved 15 November 2016.
- ↑ "US FDA Approves Once-Daily Xigduo XR Tablets for Adults with Type 2 Diabetes". AstraZeneca. 30 October 2014. Archived from the original on 16 November 2016. Retrieved 15 November 2016.
- ↑ "Xigduo XR (dapagliflozin and metformin HCl) Extended-Release Tablets". U.S. Food and Drug Administration (FDA). 7 April 2015. Archived from the original on 20 February 2020. Retrieved 5 May 2020.
- ↑ "Xigduo XR- dapagliflozin and metformin hydrochloride tablet, film coated, extended release". DailyMed. 3 February 2020. Archived from the original on 2 March 2021. Retrieved 5 May 2020.
- ↑ "Qtern EPAR". European Medicines Agency (EMA). Archived from the original on 14 July 2020. Retrieved 7 May 2020.
- ↑ "Drug Approval Package: Qtern (dapagliflozin and saxagliptin)". U.S. Food and Drug Administration (FDA). 10 October 2018. Archived from the original on 14 July 2020. Retrieved 8 May 2020.
- ↑ "Qtern- dapagliflozin and saxagliptin tablet, film coated". DailyMed. 24 January 2020. Archived from the original on 14 July 2020. Retrieved 17 February 2020.
- ↑ "Drug Approval Package: Qternmet XR". U.S. Food and Drug Administration (FDA). 27 January 2020. Archived from the original on 17 February 2020. Retrieved 17 February 2020.
- ↑ "Qtrilmet EPAR". European Medicines Agency (EMA). Archived from the original on 29 December 2019. Retrieved 30 March 2020.
- ↑ "Forxiga". Drugs.com. 4 May 2020. Archived from the original on 28 August 2021. Retrieved 5 May 2020.
- ↑ "Drug Approval Package: Farxiga (dapagliflozin) Tablets NDA #202293". U.S. Food and Drug Administration (FDA). 24 December 1999. Archived from the original on 19 September 2020. Retrieved 5 May 2020.
- ↑ "FARXIGA Granted Fast Track Designation in the US for Heart Failure Following Acute Myocardial Infarction Leveraging an Innovative Registry-Based Trial Design". www.businesswire.com. 2020-07-16. Archived from the original on 20 July 2020. Retrieved 2020-07-20.
- ↑ "Archive copy". Archived from the original on 31 August 2020. Retrieved 4 September 2020.
{{cite web}}: CS1 maint: archived copy as title (link)
External links
| External sites: | |
|---|---|
| Identifiers: |
|
- "Dapagliflozin mixture with metformin hydrochloride". Drug Information Portal. U.S. National Library of Medicine. Archived from the original on 28 August 2021. Retrieved 8 May 2020.
- "Dapagliflozin mixture with saxagliptin". Drug Information Portal. U.S. National Library of Medicine. Archived from the original on 28 August 2021. Retrieved 8 May 2020.
- Pages using duplicate arguments in template calls
- Wikipedia articles incorporating the PD-notice template
- CS1 maint: archived copy as title
- Drugs with non-standard legal status
- Chemical articles with unknown parameter in Infobox drug
- Chemical articles without CAS registry number
- Articles without EBI source
- Chemical pages without ChemSpiderID
- Chemical pages without DrugBank identifier
- Articles without KEGG source
- Articles without UNII source
- Chemicals using indexlabels
- Drugs missing an ATC code
- Drug has EMA link
- Drugboxes which contain changes to verified fields
- Drugboxes which contain changes to watched fields
- Articles with changed CASNo identifier
- Articles with changed DrugBank identifier
- Articles with changed KEGG identifier
- Anti-diabetic drugs
- AstraZeneca brands
- Bristol-Myers Squibb
- Chloroarenes
- Combination drugs
- Glucosides
- Phenol ethers
- SGLT2 inhibitors
- RTT
- World Health Organization essential medicines (alternatives)