Diclofenamide
 | |
 | |
| Names | |
|---|---|
| |
| Clinical data | |
| Drug class | Carbonic anhydrase inhibitor[1] |
| Main uses | Hyperkalemic periodic paralysis, hypokalemic periodic paralysis[1] |
| Side effects | Numbness, change in taste, confusion[1] |
| External links | |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a601233 |
| Pharmacokinetics | |
| Protein binding | 55% |
| Chemical and physical data | |
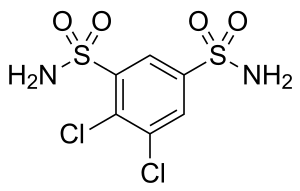
| Formula | C6H6Cl2N2O4S2 |
| Molar mass | 305.14 g·mol−1 |
| 3D model (JSmol) | |
| Melting point | 228.5 °C (443.3 °F) |
| |
| |
Diclofenamide, also known as dichlorphenamide, is a medication used to treat hyperkalemic periodic paralysis and hypokalemic periodic paralysis.[1] Evidence of benefit; however, is weak.[2] It is taken by mouth.[3] It has previously been used to treat glaucoma.[2]
Common side effects include numbness, change in taste, and confusion.[1] Other side effects may include anaphylaxis, low potassium, metabolic acidosis, and falls.[1] Safety in pregnancy is unclear.[1] It is a carbonic anhydrase inhibitor.[1]
Diclofenamide was approved for medical use in the United States in 1958.[3] They; however, were not approved for periodic paralysis until 2016.[4] It was granted orphan medicine status in Europe in 2016; however the request for approval was withdrawn in 2019.[2] In the United States 100 tablets of 50 mg costs about 26,000 USD as of 2021.[5]
Medical uses
It was originally used to treat glaucoma.[6][7][8] In 2015, the medication was approved in the US as an orphan drug for the treatment of primary hypokalemic and hyperkalemic periodic paralysis.[6][9] Evidence of benefit; however, is not strong.[2]
Dosage
For period paralysis it is started at a dose of 50 mg twice per day.[1] This may be increased up to 100 mg twice per day.[1]
Chemistry
It is a sulfonamide and a carbonic anhydrase inhibitor of the meta-disulfamoylbenzene class.
Cost
In 2001, diclofenamide had a U.S. list price of $50 for a bottle of 100 pills, and was approved for glaucoma. Merck discontinued diclofenamide when better glaucoma drugs were developed. In 2010, Sun Pharmaceutical Industries bought the rights. In 2015, the F.D.A. approved it as an orphan drug, with 7-year exclusive marketing rights, for periodic paralysis, which the company estimates affects 5,000 people in the U.S. In 2016, Strongbridge Biopharma acquired Sun, which raised the price to $15,001 for 100 pills. The cost of treatment would range from $109,500 to $219,000 a year. Sun gives the drug free to patients who don't have insurance.[9]
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 1.6 1.7 1.8 1.9 "DailyMed - KEVEYIS- dichlorphenamide tablet". dailymed.nlm.nih.gov. Archived from the original on 11 January 2022. Retrieved 24 December 2021.
- ↑ 2.0 2.1 2.2 2.3 "Ekesivy: Withdrawal of the marketing authorisation application". Archived from the original on 14 November 2021. Retrieved 24 December 2021.
- ↑ 3.0 3.1 "Dichlorphenamide Monograph for Professionals". Drugs.com. Retrieved 24 December 2021.
- ↑ "Ekesivy International non-proprietary name: diclofenamide" (PDF). Archived (PDF) from the original on 11 January 2022. Retrieved 24 December 2021.
- ↑ "Keveyis Prices, Coupons & Patient Assistance Programs". Drugs.com. Archived from the original on 26 January 2021. Retrieved 24 December 2021.
- ↑ 6.0 6.1 "Dichlorphenaide (Keveyis) for Periodic Paralysis". The Medical Letter. April 16, 2016. Archived from the original on December 22, 2017. Retrieved December 19, 2017.
- ↑ International Drug Names: Diclofenamide
- ↑ Kanski JJ (August 1968). "Carbonic anhydrase inhibitors and osmotic agents in glaucoma. Carbonic anhydrase inhibitors". The British Journal of Ophthalmology. 52 (8): 642–3. doi:10.1136/bjo.52.8.642. PMC 506660. PMID 5724852.
- ↑ 9.0 9.1 Johnson CY (December 18, 2017). "This old drug was free. Now it's $109,500 a year". Washington Post. Archived from the original on October 31, 2020. Retrieved October 19, 2020.
External links
| Identifiers: |
|
|---|
- Pages using duplicate arguments in template calls
- Chemical articles with unknown parameter in Infobox drug
- Chemical articles without CAS registry number
- Articles without EBI source
- Chemical pages without ChemSpiderID
- Chemical pages without DrugBank identifier
- Articles without KEGG source
- Articles without UNII source
- Drugs missing an ATC code
- Drugs with no legal status
- Drugboxes which contain changes to verified fields
- Drugboxes which contain changes to watched fields
- Articles with changed DrugBank identifier
- Carbonic anhydrase inhibitors
- Sulfonamides
- Chloroarenes
- RTT
- All stub articles
- Antihypertensive agent stubs