Tazarotene
 | |
 | |
| Names | |
|---|---|
| Trade names | Tazorac, Avage, Zorac, others |
| |
| Clinical data | |
| Drug class | Retinoid[1] |
| Main uses | Plaque psoriasis, acne[1] |
| Side effects | Skin redness, peeling, itchiness, skin pain[1] |
| Pregnancy category |
|
| Routes of use | Topical |
| External links | |
| AHFS/Drugs.com | Monograph |
| Legal | |
| Legal status |
|
| Pharmacokinetics | |
| Protein binding | >99% |
| Elimination half-life | 19 Hours |
| Chemical and physical data | |
| Formula | C21H21NO2S |
| Molar mass | 351.46 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Tazarotene, sold under the brand name Tazorac among others, is a medication primarily used to treat plaque psoriasis and acne.[1] It is also used for photoaged skin.[1] It is applied to the skin.[1]
Common side effects include skin redness, peeling, itchiness, and skin pain.[1] Other side effects include an increase risk of a sunburn.[1] Use in pregnancy may harm the baby while use during breastfeeding appear okay.[1][2] It is a third-generation retinoid of the acetylenic class.[3][1]
Tazarotene was approved for medical use in 1997.[1] It is available as a generic medication.[4] In the United States a 30 gram tube of 0.1% costs about 70 USD as of 2021.[4] This amount in the United Kingdom costs the NHS about 15 pounds.[5] It is also available in some other European countries as of 2021.[2]
Medical uses
Tazarotene is most commonly used topically to treat acne vulgaris and psoriasis.[6] Like other topical retinoids, such as tretinoin and adapalene, tazarotene can be combined with benzoyl peroxide or an oral antibiotic, such as clindamycin or dapsone, for the treatment of acne.[7] This results in increased efficacy compared to tazarotene monotherapy.[7] For psoriasis, a combination therapy of tazarotene and a mid- to high-potency corticosteroid is more effective than either treatment alone.[8]
Tazarotene can also be used for the treatment of photodamaged skin. It can reduce the clinical and histological signs of photodamaged skin.[9] The therapy is more effective when used with the daily application of sunscreen.[10]
Contraindications
Tazarotene is contraindicated for use in patients who are known to be or suspected of being pregnant.[6] It is also contraindicated in patients with a known hypersensitivity to any ingredient in the specific pharmaceutical formulation.[6]
Pregnancy
Before 2015, tazarotene was considered a Category X drug (meaning its use was contraindicated during pregnancy) according to Food and Drug Administration (FDA) guidelines, despite demonstrating similar plasma retinoid levels as adapalene and tretinoin, which were classified as Category C drugs.[11] Under the FDA's updated Pregnancy and Lactation Labeling Rule which eliminated the lettered pregnancy categories and came into effect in 2015, tazarotene was determined to be contraindicated in pregnancy.[11] Because of the lack of pregnancy outcomes data for the drug, the determination was based on the teratogenic effects observed in rat and rabbit studies.[12][11]
Side effects
Side effects for tazarotene include skin irritation, such as redness, itchiness, and burning. In patients with psoriasis, these adverse effects can be mitigated by a combined treatment with either mometasone furoate or fluocinonide.[8] These effects tend to be mild to moderate, and increase in intensity as tazarotene concentration increases.[13]
Pharmacology
Mechanism of action
Tazarotene is selective for two types of retinoic acid receptors, RAR-γ and RAR-β.[14] Like all retinoids, it affects the ability of keratinocytes in the epidermis to proliferate and differentiate.[14] It does so by upregulating filaggrin expression and downregulating the expression of keratinocyte transglutaminase, ornithine decarboxylase, involucrin, epidermal growth factor receptor, and various keratins.[15]
Pharmacokinetics
More than 99% of tazarotenic acid, the active metabolite of tazarotene, in the blood binds to plasma proteins (the most predominant being albumin).[16] The volume of distribution (VD) for tazarotene is 26.1 L/kg and the VD for tazarotenic acid is 1.97 L/kg.[16] Tazarotene is excreted from the body via feces and urine equally,[8] and it has an elimination half-life of 16[17] to 18 hours.[18]
Society and culture
Synthesis
Acetylenic retinoid prodrug converted to the active metabolite, tazarotenic acid, with selective affinity for retinoic acid receptors RARβ and RARγ.
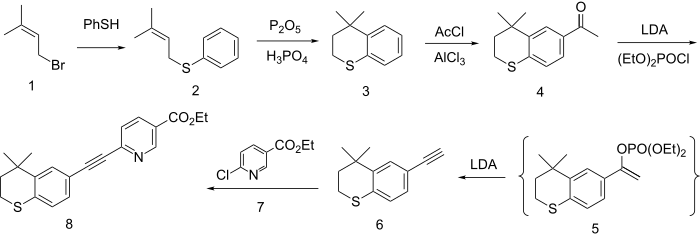
The formation of the ring system involves first alkylation of the anion from thiophenol with dimethylallyl bromide (1) to give the thioether (2). Friedel-Crafts cyclization of the olefin with the equivalent of PPA then gives the thiopyran (3). Acylation with acetyl chloride in the presence of aluminium chloride gives the methyl ketone (4). Reaction of the enolate of that ketone with diethyl chlorophosphate gives the enol phosphate 5 as a transient intermediate. This eliminates diethyl phosphite in the presence of excess base to give the corresponding acetylene 6. The anion from the reaction of the acetylene with base is then used to displace chlorine from Ethyl 6-chloronicotinate (7). This reaction affords the coupling product tazarotene (8).
References
- ↑ 1.00 1.01 1.02 1.03 1.04 1.05 1.06 1.07 1.08 1.09 1.10 "Tazarotene Monograph for Professionals". Drugs.com. Archived from the original on January 22, 2021. Retrieved September 24, 2021.
- ↑ 2.0 2.1 "Tazarotene". SPS - Specialist Pharmacy Service. April 28, 2015. Archived from the original on September 24, 2021. Retrieved September 24, 2021.
- ↑ Thielitz A, Abdel-Naser MB, Fluhr JW, Zouboulis CC, Gollnick H (December 2008). "Topical retinoids in acne--an evidence-based overview". J Dtsch Dermatol Ges. 6 (12): 1023–31. doi:10.1111/j.1610-0387.2008.06741.x. PMID 18479477.
- ↑ 4.0 4.1 "Tazarotene Prices, Coupons & Savings Tips - GoodRx". GoodRx. Retrieved September 24, 2021.
- ↑ BNF (80 ed.). BMJ Group and the Pharmaceutical Press. September 2020 – March 2021. p. 1330. ISBN 978-0-85711-369-6.
- ↑ 6.0 6.1 6.2 "Tazarotene Monograph for Professionals". Drugs.com. American Society of Health-System Pharmacists. Archived from the original on January 22, 2021. Retrieved March 11, 2021.
- ↑ 7.0 7.1 Kolli SS, Pecone D, Pona A, Cline A, Feldman SR (June 2019). "Topical Retinoids in Acne Vulgaris: A Systematic Review". Am J Clin Dermatol. 20 (3): 345–365. doi:10.1007/s40257-019-00423-z. PMID 30674002.
- ↑ 8.0 8.1 8.2 Foster RH, Brogden RN, Benfield P (May 1998). "Tazarotene". Drugs. 55 (5): 705–11, discussion 712. doi:10.2165/00003495-199855050-00008. PMID 9585866.
- ↑ Stratigos AJ, Katsambas AD (2005). "The role of topical retinoids in the treatment of photoaging". Drugs. 65 (8): 1061–72. doi:10.2165/00003495-200565080-00003. PMID 15907143.
- ↑ Ogden S, Samuel M, Griffiths CE (2008). "A review of tazarotene in the treatment of photodamaged skin". Clin Interv Aging. 3 (1): 71–6. doi:10.2147/cia.s1101. PMC 2544371. PMID 18488880.
- ↑ 11.0 11.1 11.2 Han G, Wu JJ, Del Rosso JQ (September 2020). "Use of Topical Tazarotene for the Treatment of Acne Vulgaris in Pregnancy: A Literature Review". J Clin Aesthet Dermatol. 13 (9): E59–E65. PMC 7577328. PMID 33133344.
- ↑ "Arazlo (tazarotene) [package insert]" (PDF). Drugs@FDA. U.S. Food and Drug Administration. Archived (PDF) from the original on April 11, 2021. Retrieved March 11, 2021.
- ↑ Mukherjee S, Date A, Patravale V, Korting HC, Roeder A, Weindl G (2006). "Retinoids in the treatment of skin aging: an overview of clinical efficacy and safety". Clin Interv Aging. 1 (4): 327–48. doi:10.2147/ciia.2006.1.4.327. PMC 2699641. PMID 18046911.
- ↑ 14.0 14.1 Duvic M, Nagpal S, Asano AT, Chandraratna RA (August 1997). "Molecular mechanisms of tazarotene action in psoriasis". J Am Acad Dermatol. 37 (2 Pt 3): S18–24. PMID 9270552.
- ↑ Heath MS, Sahni DR, Curry ZA, Feldman SR (September 2018). "Pharmacokinetics of tazarotene and acitretin in psoriasis". Expert Opin Drug Metab Toxicol. 14 (9): 919–927. doi:10.1080/17425255.2018.1515198. PMID 30134735.
- ↑ 16.0 16.1 Tang-Liu DD, Matsumoto RM, Usansky JI (October 1999). "Clinical pharmacokinetics and drug metabolism of tazarotene: a novel topical treatment for acne and psoriasis". Clin Pharmacokinet. 37 (4): 273–87. doi:10.2165/00003088-199937040-00001. PMID 10554045.
- ↑ Marks R (October 1998). "Pharmacokinetics and safety review of tazarotene". J Am Acad Dermatol. 39 (4 Pt 2): S134–8. doi:10.1016/s0190-9622(98)70310-x. PMID 9777791.
- ↑ Menter A (August 2000). "Pharmacokinetics and safety of tazarotene". J Am Acad Dermatol. 43 (2 Pt 3): S31–5. doi:10.1067/mjd.2000.108321. PMID 10898827.
- ↑ Prepn: Chandraratna RA, EP 284288; idem, U.S. Patent 5,089,509 (1988, 1992 both to Allergan).
External links
| Identifiers: |
|
|---|
- Pages using duplicate arguments in template calls
- Use mdy dates from March 2021
- Articles with invalid date parameter in template
- Drugs with non-standard legal status
- Chemical articles with unknown parameter in Infobox drug
- Chemical articles without CAS registry number
- Articles without EBI source
- Chemical pages without ChemSpiderID
- Chemical pages without DrugBank identifier
- Articles without KEGG source
- Articles without UNII source
- Drugs missing an ATC code
- Retinoids
- Phenylacetylenes
- Nicotinate esters
- Anti-acne preparations
- Antipsoriatics
- Ethyl esters
- RTT