Avanafil
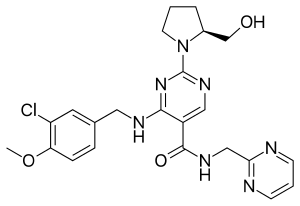 | |
 Avanafil is a PDE5 inhibitor | |
| Names | |
|---|---|
| Trade names | Stendra |
| |
| Clinical data | |
| Drug class | PDE5 inhibitor[1] |
| Main uses | Erectile dysfunction[1] |
| Side effects | Headache, flushing, stuffy nose, back pain[1] |
| Interactions | Nitrovasodilator[1] |
| Pregnancy category |
|
| Routes of use | By mouth |
| Onset of action | Within 15 min[2] |
| Duration of action | 2 hr[2] |
| Typical dose | 50 to 200 mg[2] |
| External links | |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a614010 |
| Legal | |
| License data | |
| Legal status | |
| Chemical and physical data | |
| Formula | C23H26ClN7O3 |
| Molar mass | 483.96 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Avanafil, sold under the brand names Stendra and Spedra, is medication used to treat erectile dysfunction.[1] It is taken by mouth.[3] Effects generally begin within 15 minutes and last for up to 2 hours.[2]
Common side effects include headache, flushing, stuffy nose, and back pain.[1] Other side effects may include priapism, vision loss, and hearing loss.[3] It should not be used with nitrovasodilator.[1] It should also be avoided in people with significant liver or kidney problems.[3] It is a PDE5 inhibitor and works by blocking the phosphodiesterase enzyme, thus increasing levels of cyclic guanosine monophosphate (cGMP).[1]
Avanafil was approved for medical use in the United States in 2012 and Europe in 2013.[3][1] In the United Kingdom 4 pills of 100 mg costs the NHS about £14 as of 2021.[4] This amount in the United States is about 210 USD.[5]
Medical use
Dosage
It is taken at a dose of 100 mg about 15 minutes before sex.[3]
Mechanism of action
Avanafil acts by inhibiting a specific phosphodiesterase type 5 enzyme found in various body tissues, primarily in the corpus cavernosum penis.[6] Other similar drugs are sildenafil, tadalafil and vardenafil. The advantage of avanafil is that it has very fast onset of action compared with other PDE5 inhibitors. It is absorbed quickly, reaching a maximum serum concentration in about thirty to forty-five minutes.[7]
Synthesis
Avanafil can be synthesized from a benzylamine derivative and a pyrimidine derivative:[8]
History
It was invented at Mitsubishi Tanabe Pharma, formerly known as Tanabe Seiyaku Co.,[8] and licensed to Vivus Inc., which partnered with Menarini Group to commercialise Spedra in over forty European countries, Australia, and New Zealand.[9] Metuchen Pharmaceuticals obtained exclusive rights within the United States.[10]
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 1.6 1.7 1.8 "Spedra". Archived from the original on 11 April 2021. Retrieved 16 January 2022.
- ↑ 2.0 2.1 2.2 2.3 "Avanafil". American Society of Health-System Pharmacists. Archived from the original on 30 November 2020. Retrieved 16 January 2022.
- ↑ 3.0 3.1 3.2 3.3 3.4 "DailyMed - STENDRA- avanafil tablet". dailymed.nlm.nih.gov. Archived from the original on 17 April 2021. Retrieved 16 January 2022.
- ↑ BNF 81: March-September 2021. BMJ Group and the Pharmaceutical Press. 2021. p. 859. ISBN 978-0857114105.
- ↑ "Stendra Prices, Coupons & Savings Tips - GoodRx". GoodRx. Archived from the original on 15 October 2021. Retrieved 16 January 2022.
- ↑ "avanafil, Spedra". Medicine Net. Archived from the original on 20 April 2014. Retrieved 17 April 2014.
- ↑ Kyle JA, Brown DA, Hill JK (October 2013). "Avanafil for erectile dysfunction". The Annals of Pharmacotherapy. Sage Publishing. 47 (10): 1312–20. doi:10.1177/1060028013501989. PMID 24259695. S2CID 6562049.
- ↑ 8.0 8.1 US 6797709, Yamada K, Matsuki K, Omori K Kikkawa K, "Aromatic nitrogen-containig 6-membered cyclic compounds", issued 11 December 2003, assigned to Tanabe Seiyaku Co
- ↑ "VIVUS Announces Avanafil Partnership With Menarini". Vivus Inc. Archived from the original on 2015-12-08.
- ↑ "VIVUS and Metuchen Pharmaceuticals Announce License Agreement for Commercial Rights to Stendra". Vivus Inc. 3 October 2016. Archived from the original on 10 September 2019. Retrieved 17 April 2021.
External links
| External sites: | |
|---|---|
| Identifiers: |
|
- Pages using duplicate arguments in template calls
- Drugs with non-standard legal status
- Chemical articles with unknown parameter in Infobox drug
- Chemical articles without CAS registry number
- Articles without EBI source
- Chemical pages without ChemSpiderID
- Chemical pages without DrugBank identifier
- Articles without KEGG source
- Articles without UNII source
- Drugs missing an ATC code
- Drug has EMA link
- Drugboxes which contain changes to verified fields
- Drugboxes which contain changes to watched fields
- Articles with changed EBI identifier
- Primary alcohols
- Carboxamides
- Chloroarenes
- PDE5 inhibitors
- Phenol ethers
- Aminopyrimidines
- Pyrrolidines
- Erectile dysfunction drugs
- RTT
- Enones
