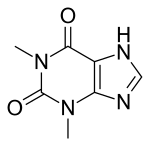
Theophylline
 | |
 | |
| Names | |
|---|---|
| Trade names | Theolair, Slo-Bid, others |
| Other names | 1,3-dimethylxanthine |
| |
| Clinical data | |
| Pregnancy category |
|
| Routes of use | By mouth, IV[2] |
| External links | |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a681006 |
| Legal | |
| Legal status |
|
| Pharmacokinetics | |
| Bioavailability | 100% (IV) |
| Protein binding | 40% (primarily to albumin) |
| Metabolism | Liver to 1-methyluric acid |
| Elimination half-life | 5–8 hours |
| Chemical and physical data | |
| Formula | C7H8N4O2 |
| Molar mass | 180.167 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Theophylline, sold under various brand names, is a medication used for chronic obstructive pulmonary disease (COPD) and asthma.[2] Use has largely been replaced by other bronchodilators with greater safety.[3] It is used by mouth or by injection into a vein.[2] Theophylline blood levels require periodic monitoring.[4]
Common side effects include nausea, headache, trouble sleeping, abdominal pain, palpitations, and diarrhea.[2] Severe side effects may include low blood potassium.[4] Excessive doses can result in severe side effects.[4] The dose may need to be adjusted depending on if someone smokes, uses alcohol, or has liver or heart problems.[4] Use during pregnancy is of unclear safety.[1] It is in the xanthine family of medications.[2] How it works is not entirely clear.[2]
Theophylline was isolated in 1888 from the tea plant (Camellia sinensis).[3] It is available as a generic medication.[2] In the United Kingdom a month of medication costs the NHS about 5 pounds.[4]
Medical uses
Uses of theophylline are include chronic obstructive pulmonary disease (COPD) and asthma.[2] Such use has largely been replaced by other bronchodilators with greater safety.[3]
Apnea of prematurity; however, caffeine is preferred for this use[2][5]
Side effects
The use of theophylline is complicated by its interaction with various drugs and by the fact that it has a narrow therapeutic window. Its use must be monitored by direct measurement of serum theophylline levels to avoid toxicity. It can also cause nausea, diarrhea, increase in heart rate, abnormal heart rhythms, and CNS excitation (headaches, insomnia, irritability, dizziness and lightheadedness).[6][7] Seizures can also occur in severe cases of toxicity, and are considered to be a neurological emergency.[8] Its toxicity is increased by erythromycin, cimetidine, and fluoroquinolones, such as ciprofloxacin. Some lipid-based formulations of theophylline can result in toxic theophylline levels when taken with fatty meals, an effect called dose dumping, but this does not occur with most formulations of theophylline.[9] Theophylline toxicity can be treated with beta blockers. In addition to seizures, tachyarrhythmias are a major concern.[10] Theophylline should not be used in combination with the SSRI fluvoxamine.[11][12][13]
Pharmacology

The main actions of theophylline involve:
- relaxing bronchial smooth muscle
- increasing heart muscle contractility and efficiency (positive inotrope)
- increasing heart rate (positive chronotropic)[15]
- increasing blood pressure
- increasing kidney blood flow
- anti-inflammatory effects
- central nervous system stimulatory effect mainly on the medullary respiratory center.
- Blocks the action of adenosine; an inhibitory neurotransmitter that induces sleep, contracts the smooth muscles and relaxes the cardiac muscle.
Pharmacodynamics
Like other methylated xanthine derivatives, theophylline is both a
- competitive nonselective phosphodiesterase inhibitor,[16] which raises intracellular cAMP, activates PKA, inhibits TNF-alpha[17][18] and inhibits leukotriene[19] synthesis, and reduces inflammation and innate immunity[19]
- nonselective adenosine receptor antagonist,[20] antagonizing A1, A2, and A3 receptors almost equally, which explains many of its cardiac effects
Theophylline has been shown to inhibit TGF-beta-mediated conversion of pulmonary fibroblasts into myofibroblasts in COPD and asthma via cAMP-PKA pathway and suppresses COL1 mRNA, which codes for the protein collagen.[21]
It has been shown that theophylline may reverse the clinical observations of steroid insensitivity in patients with COPD and asthmatics who are active smokers (a condition resulting in oxidative stress) via a distinctly separate mechanism. Theophylline in vitro can restore the reduced HDAC (histone deacetylase) activity that is induced by oxidative stress (i.e., in smokers), returning steroid responsiveness toward normal.[22] Furthermore, theophylline has been shown to directly activate HDAC2.[22] (Corticosteroids switch off the inflammatory response by blocking the expression of inflammatory mediators through deacetylation of histones, an effect mediated via histone deacetylase-2 (HDAC2). Once deacetylated, DNA is repackaged so that the promoter regions of inflammatory genes are unavailable for binding of transcription factors such as NF-κB that act to turn on inflammatory activity. It has recently been shown that the oxidative stress associated with cigarette smoke can inhibit the activity of HDAC2, thereby blocking the anti-inflammatory effects of corticosteroids.)
Pharmacokinetics
Absorption
When theophylline is administered intravenously, bioavailability is 100%. [23]
Distribution
Theophylline is distributed in the extracellular fluid, in the placenta, in the mother's milk and in the central nervous system. The volume of distribution is 0.5 L/kg. The protein binding is 40%. The volume of distribution may increase in neonates and those suffering from cirrhosis or malnutrition, whereas the volume of distribution may decrease in those who are obese.
Metabolism
Theophylline is metabolized extensively in the liver (up to 70%). It undergoes N-demethylation via cytochrome P450 1A2. It is metabolized by parallel first order and Michaelis-Menten pathways. Metabolism may become saturated (non-linear), even within the therapeutic range. Small dose increases may result in disproportionately large increases in serum concentration. Methylation to caffeine is also important in the infant population. Smokers and people with hepatic (liver) impairment metabolize it differently. Both THC and nicotine have been shown to increase the rate of theophylline metabolism.[24]
Excretion
Theophylline is excreted unchanged in the urine (up to 10%). Clearance of the drug is increased in children (age 1 to 12), teenagers (12 to 16), adult smokers, elderly smokers, as well as in cystic fibrosis, and hyperthyroidism. Clearance of the drug is decreased in these conditions: elderly, acute congestive heart failure, cirrhosis, hypothyroidism and febrile viral illnesses.
The elimination half-life varies: 30 hours for premature neonates, 24 hours for neonates, 3.5 hours for children ages 1 to 9, 8 hours for adult non-smokers, 5 hours for adult smokers, 24 hours for those with hepatic impairment, 12 hours for those with congestive heart failure NYHA class I-II, 24 hours for those with congestive heart failure NYHA class III-IV, 12 hours for the elderly.
Spectroscopy
On UV-visible spectroscopy theophylline is soluble in 0.1N NaOH and absorbs maximally at 277 nm with an extinction coefficient of 10,200 (cm−1/M).[25]
On proton nuclear magnetic resonance spectroscopy (1H-NMR) the characteristic signals, distinguishing theophylline from related methylxanthines, are approximately 3.23δ and 3.41δ, corresponding to the unique methylation possessed by theophylline. The remaining proton signal, at 8.01δ, corresponds to the proton on the imidazole ring, not transferred between the nitrogen. The transferred proton between the nitrogen is a variable proton and only exhibits a signal under certain conditions.[26]
On carbon nuclear magnetic resonance spectroscopy (13C-NMR) the unique methylation of theophylline corresponds to the following signals: 27.7δ and 29.9δ. The remaining signals correspond to carbons characteristic of the xanthine backbone.[27]
Natural occurrences
Theophylline is naturally found in cocoa beans. Amounts as high as 3.7 mg/g have been reported in Criollo cocoa beans.[28]
Trace amounts of theophylline are also found in brewed tea, although brewed tea provides only about 1 mg/l,[29] which is less than a therapeutic dose.
Trace amounts of theophylline are also found in guarana (Paullinia cupana) and in kola nuts cola (plant) [30] A small amount of theophylline is one of the products of caffeine metabolic processing in the liver.[31]
History

Theophylline was first extracted from tea leaves and chemically identified around 1888 by the German biologist Albrecht Kossel.[32][33] Seven years later, a chemical synthesis starting with 1,3-dimethyluric acid was described by Emil Fischer and Lorenz Ach.[34] The Traube purine synthesis, an alternative method to synthesize theophylline, was introduced in 1900 by another German scientist, Wilhelm Traube.[35] Theophylline's first clinical use came in 1902 as a diuretic.[36] It took an additional 20 years until it was first reported as an asthma treatment.[37] The drug was prescribed in a syrup up to the 1970s as Theostat 20 and Theostat 80, and by the early 1980s in a tablet form called Quibron.
Research
Current research regarding theophylline-based therapies are oriented towards employing theophylline, as well as other methylxanthines as natural scaffolding for new bronchodilatory pharmaceuticals. In 2017, Mohammed, et al.[38] demonstrated potential derivitization of methylxanthines to produce significant broncodilatory effects in a small mouse study group. However, additional work is oriented towards limiting the interactions of theophylline-based therapies with other drugs and structures, as theophylline has been demonstrated as an effective adjunctive therapy[39] in various diseases. These interactions include maintaining, for example, its activity with HDAC, but eliminating its stimulatory behavior (or rather its adenosine-antagonist behavior).[40]
References
- ↑ 1.0 1.1 "Theophylline Use During Pregnancy". Drugs.com. Archived from the original on 29 November 2020. Retrieved 9 October 2020.
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 2.6 2.7 2.8 "Theophyllines Monograph for Professionals". Drugs.com. Archived from the original on 23 September 2020. Retrieved 9 October 2020.
- ↑ 3.0 3.1 3.2 Sneader, Walter (2005). Drug Discovery: A History. John Wiley & Sons. p. 95. ISBN 978-0-471-89979-2. Archived from the original on 2021-08-29. Retrieved 2020-10-09.
- ↑ 4.0 4.1 4.2 4.3 4.4 BNF 79 : March 2020. London: Royal Pharmaceutical Society. 2020. p. 282. ISBN 9780857113658.
- ↑ Eichenwald, Eric C. (January 2016). "Apnea of Prematurity". Pediatrics. 137 (1): e20153757. doi:10.1542/peds.2015-3757.
- ↑ MedlinePlus Drug Information: Theophylline Archived 2016-07-05 at the Wayback Machine
- ↑ THEOPHYLLINE - ORAL 24 HOUR TABLET (Uni-Dur) side effects, medical uses, and drug interactions Archived 2008-05-09 at the Wayback Machine
- ↑ Yoshikawa H (Apr 2007). "First-line therapy for theophylline-associated seizures". Acta Neurol Scand. 115 (4 Suppl): 57–61. doi:10.1111/j.1600-0404.2007.00810.x. PMID 17362277. S2CID 11347304.
- ↑ Hendeles L, Weinberger M, Milavetz G, Hill M, Vaughan L (1985). "Food-induced "dose-dumping" from a once-a-day theophylline product as a cause of theophylline toxicity". Chest. 87 (6): 758–65. doi:10.1378/chest.87.6.758. PMID 3996063. S2CID 1133968. Archived from the original on 2021-08-29. Retrieved 2019-12-16.
- ↑ Seneff M, Scott J, Friedman B, Smith M (1990). "Acute theophylline toxicity and the use of esmolol to reverse cardiovascular instability". Annals of Emergency Medicine. 19 (6): 671–3. doi:10.1016/s0196-0644(05)82474-6. PMID 1971502.
- ↑ Devane, C. L.; Markowitz, J. S.; Hardesty, S. J.; Mundy, S.; Gill, H. S. (September 1997). "Fluvoxamine-induced theophylline toxicity". American Journal of Psychiatry. 154 (9): 1317b–1318. doi:10.1176/ajp.154.9.1317b. ISSN 0002-953X. PMID 9286199.
- ↑ Sperber, A. D. (November 1991). "Toxic interaction between fluvoxamine and sustained release theophylline in an 11-year-old boy". Drug Safety. 6 (6): 460–462. doi:10.2165/00002018-199106060-00006. ISSN 0114-5916. PMID 1793525. S2CID 21875026.
- ↑ Brøsen, K. (September 1998). "Differences in interactions of SSRIs". International Clinical Psychopharmacology. 13 Suppl 5: S45–47. doi:10.1097/00004850-199809005-00009. ISSN 0268-1315. PMID 9817620. S2CID 38403377.
- ↑ Barnes, Peter J. (March 2010). "Theophylline". Pharmaceuticals. 3 (3): 725–747. doi:10.3390/ph3030725. ISSN 1424-8247.
- ↑ Alboni et al. Effects of Permanent Pacemaker and Oral Theophylline in Sick Sinus Syndrome The THEOPACE Study: A Randomized Controlled Trial Archived 2013-08-02 at Wikiwix
- ↑ Essayan DM (2001). "Cyclic nucleotide phosphodiesterases". J Allergy Clin Immunol. 108 (5): 671–80. doi:10.1067/mai.2001.119555. PMID 11692087.
- ↑ Deree J, Martins JO, Melbostad H, Loomis WH, Coimbra R (2008). "Insights into the Regulation of TNF-α Production in Human Mononuclear Cells: The Effects of Non-Specific Phosphodiesterase Inhibition". Clinics (Sao Paulo). 63 (3): 321–8. doi:10.1590/S1807-59322008000300006. PMC 2664230. PMID 18568240.
- ↑ Marques LJ, Zheng L, Poulakis N, Guzman J, Costabel U (February 1999). "Pentoxifylline inhibits TNF-alpha production from human alveolar macrophages". Am. J. Respir. Crit. Care Med. 159 (2): 508–11. doi:10.1164/ajrccm.159.2.9804085. PMID 9927365.
- ↑ 19.0 19.1 Peters-Golden M, Canetti C, Mancuso P, Coffey MJ (2005). "Leukotrienes: underappreciated mediators of innate immune responses". J. Immunol. 174 (2): 589–94. doi:10.4049/jimmunol.174.2.589. PMID 15634873.
- ↑ Daly JW, Jacobson KA, Ukena D (1987). "Adenosine receptors: development of selective agonists and antagonists". Prog Clin Biol Res. 230 (1): 41–63. PMID 3588607.
- ↑ Yano Y, Yoshida M, Hoshino S, Inoue K, Kida H, Yanagita M, Takimoto T, Hirata H, Kijima T (2006). "Anti-fibrotic effects of theophylline on lung fibroblasts". Biochemical and Biophysical Research Communications. 341 (3): 684–90. doi:10.1016/j.bbrc.2006.01.018. PMID 16430859.
- ↑ 22.0 22.1 Ito K, Lim S, Caramori G, Cosio B, Chung KF, Adcock IM, Barnes PJ (2002). "A molecular mechanism of action of theophylline: Induction of histone deacetylase activity to decrease inflammatory gene expression". Proceedings of the National Academy of Sciences of the United States of America. 99 (13): 8921–6. doi:10.1073/pnas.132556899. PMC 124399. PMID 12070353.
- ↑ Griffin, J. P. The Textbook of Pharmaceutical Medicine (6th ed.). New Jersey: BMJ Books. ISBN 978-1-4051-8035-1
- ↑ "RxList Marinol Interactions". 2008-05-29. Archived from the original on 2013-07-06. Retrieved 2013-06-02. (accessdate may be 2013-02-06)
- ↑ Schack, Jerome A., and Samuel H. Waxler. "An ultraviolet spectrophotometric method for the determination of theophylline and theobromine in blood and tissues." Journal of pharmacology and experimental therapeutics 97.3 (1949): 283-291.
- ↑ Shelke, R. U.; Degani, M. S.; Raju, A.; Ray, M. K.; Rajan, M. G. R. Fragment Discovery for the Design of Nitrogen Heterocycles as Mycobacterium tuberculosis Dihydrofolate Reductase Inhibitors. Archiv der Pharmazie 2016, 349, 602-613.
- ↑ Pfleiderer, W. Pteridines. Part CXIX. Helvetica Chimica Acta 2008, 91, 338-353.
- ↑ Apgar, Joan L; Tarka, Stanly M. Jr. (1998). "Methylxanthine composition and consumption patterns of cocoa and chocolate products and their uses". In Gene A. Spiller (ed.). Caffeine. CRC Press. p. 171. ISBN 978-0-8493-2647-9. Retrieved 2013-11-10.
- ↑ MAFF Food Surveillance Information Sheet Archived 2006-09-27 at the Wayback Machine
- ↑ Belliardo F, Martelli A, Valle MG. HPLC determination of caffeine and theophylline in Paullinia cupana Kunth (guarana) and Cola spp. samples. Z Lebensm Unters Forsch. 1985 May;180(5):398-401.
- ↑ Mandal, Ananya. "Caffeine Pharmacology". Website Medical News. Archived from the original on 2016-05-31.
- ↑ Kossel A (1888). "Über eine neue Base aus dem Pflanzenreich". Ber. Dtsch. Chem. Ges. 21: 2164–2167. doi:10.1002/cber.188802101422. Archived from the original on 2021-11-04. Retrieved 2020-09-06.
- ↑ Kossel A (1889). "Über das Theophyllin, einen neuen Bestandtheil des Thees". Hoppe-Seyler's Z. Physiol. Chem. 13: 298–308.
- ↑ Fischer E, Ach L (1895). "Synthese des Caffeins". Ber. Dtsch. Chem. Ges. 28 (3): 3139. doi:10.1002/cber.189502803156.
- ↑ Traube W (1900). "Der synthetische Aufbau der Harnsäure, des Xanthins, Theobromins, Theophyllins und Caffeïns aus der Cyanessigsäure]". Chem. Ber. 33 (3): 3035–3056. doi:10.1002/cber.19000330352. Archived from the original on 2021-03-08. Retrieved 2019-06-28.
- ↑ Minkowski O (1902). "Über Theocin (Theophyllin) als Diureticum". Ther. Gegenwart. 43: 490–493.
- ↑ Schultze-Werninghaus G, Meier-Sydow J (1982). "The clinical and pharmacological history of theophylline: first report on the bronchospasmolytic action in man by S. R. Hirsch in Frankfurt (Main) 1922". Clin. Allergy. 12 (2): 211–215. doi:10.1111/j.1365-2222.1982.tb01641.x. PMID 7042115.
- ↑ Mohamed, A. R.; Georgey, H. H.; George, R. F.; El-Eraky, W. I.; Saleh, D. O.; Abdel Gawad, N. M. Identification of some novel xanthine-based derivatives with bronchodilator activity. Future Medicinal Chemistry 2017, 9, 1731-1747.
- ↑ João Monteiro; Marco G Alves; Pedro F Oliveira; Branca M Silva Pharmacological potential of methylxanthines: Retrospective analysis and future expectations. Critical reviews in food science and nutrition 2018, 58, 1-29.
- ↑ Barnes, P. J. Theophylline in Chronic Obstructive Pulmonary Disease: New Horizons. Proceedings of the American Thoracic Society 2005, 2, 1.
External links
| Identifiers: |
|
|---|
- Pages using duplicate arguments in template calls
- Webarchive template wayback links
- CS1: long volume value
- Webarchive template other archives
- Chemical articles with unknown parameter in Infobox drug
- Chemical articles without CAS registry number
- Articles without EBI source
- Chemical pages without ChemSpiderID
- Chemical pages without DrugBank identifier
- Articles without KEGG source
- Articles without UNII source
- Drugs missing an ATC code
- Drugboxes which contain changes to watched fields
- Adenosine receptor antagonists
- Beta1-adrenergic agonists
- Bitter compounds
- Merck brands
- Bronchodilators
- Phosphodiesterase inhibitors
- Xanthines
- Histone Acetyltransferase Inhibitor
- RTT