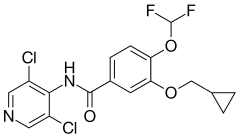
Roflumilast
 | |
 drugb | |
| Names | |
|---|---|
| Trade names | Daxas, Daliresp, others |
| |
| Clinical data | |
| Drug class | Phosphodiesterase-4 inhibitor (PDE-4)[1] |
| Main uses | Chronic obstructive pulmonary disease (COPD)[1] |
| Side effects | Diarrhea, nausea, headache, back pain, trouble sleeping, dizziness[2] |
| Pregnancy category |
|
| Routes of use | By mouth |
| Typical dose | 0.5 mg OD[1] |
| External links | |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a611034 |
| Legal | |
| License data |
|
| Legal status | |
| Pharmacokinetics | |
| Bioavailability | 79%[5][4][6][7] |
| Protein binding | 99%[5][4][6][7] |
| Metabolism | Liver via CYP1A2 & CYP3A4[5][4][6][7] |
| Elimination half-life | 17 hours (30 hours [active metabolite])[5][4][6][7] |
| Excretion | Urine (70%)[5][4][6][7] |
| Chemical and physical data | |
| Formula | C17H14Cl2F2N2O3 |
| Molar mass | 403.21 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Roflumilast, sold under the trade name Daxas among others, is a medication used for the long term management of chronic obstructive pulmonary disease (COPD).[1] It is used in those with severe disease.[1] It is taken by mouth.[1]
Common side effects include diarrhea, nausea, headache, back pain, trouble sleeping, and dizziness.[2] Other side effects can include suicide.[2] It is a selective inhibitor of the enzyme phosphodiesterase-4 (PDE-4) and works by decreasing lung inflammation.[1][3]
Roflumilast was approved for medical use in Europe in 2010 and the United States in 2011.[3][8][2] In the United Kingdom it costs the NHS about £38 per month as of 2021.[9] This amount in the United States costs about 430 USD.[10]
Medical uses
Its primary use is in the prevention of exacerbations in severe chronic obstructive pulmonary disease (COPD).[5][4][6][7][3] It is however unclear if benefits are greater than harms.[11]
Dosage
It is taken at a dose of 0.5 mg per day.[1] Some people may be started at half the that dose for the first month.[2]
Side effects
Common (1–10% incidence) adverse effects include:[5][4][6][7][12]
- Diarrhea
- Weight loss
- Nausea
- Headache
- Insomnia
- Decreased appetite
- Abdominal pain
- Rhinitis
- Sinusitis
- Urinary tract infection
- Depression
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 1.6 1.7 "Roflumilast Monograph for Professionals". Drugs.com. Archived from the original on 25 February 2021. Retrieved 18 October 2021.
- ↑ 2.0 2.1 2.2 2.3 2.4 "DailyMed - DALIRESP- roflumilast tablet". dailymed.nlm.nih.gov. Archived from the original on 25 March 2021. Retrieved 18 October 2021.
- ↑ 3.0 3.1 3.2 3.3 "Daxas EPAR". European Medicines Agency. Archived from the original on 12 August 2020. Retrieved 28 September 2020.
- ↑ 4.0 4.1 4.2 4.3 4.4 4.5 4.6 4.7 "Daxas 250 micrograms tablets - Summary of Product Characteristics (SmPC)". (emc). 11 June 2020. Archived from the original on 19 September 2020. Retrieved 28 September 2020.
- ↑ 5.0 5.1 5.2 5.3 5.4 5.5 5.6 5.7 "Daliresp- roflumilast tablet". DailyMed. 12 March 2020. Archived from the original on 25 March 2021. Retrieved 28 September 2020.
- ↑ 6.0 6.1 6.2 6.3 6.4 6.5 6.6 "Daliresp : EPAR - Product Information" (PDF). European Medicines Agency. Takeda GmbH. 26 September 2013. Archived (PDF) from the original on 26 June 2016. Retrieved 18 November 2013.
- ↑ 7.0 7.1 7.2 7.3 7.4 7.5 7.6 "roflumilast (Rx) - Daliresp". Medscape Reference. WebMD. Archived from the original on 12 September 2017. Retrieved 18 November 2013.
- ↑ ""Nycomed's Anti-Inflammatory Gains Approval in EU for COPD"". Archived from the original on 2017-08-24. Retrieved 2021-07-21.
- ↑ BNF (80 ed.). BMJ Group and the Pharmaceutical Press. September 2020 – March 2021. p. 286. ISBN 978-0-85711-369-6.
{{cite book}}: CS1 maint: date format (link) - ↑ "Daliresp Prices, Coupons & Patient Assistance Programs". Drugs.com. Archived from the original on 11 April 2021. Retrieved 18 October 2021.
- ↑ Ton, Joey (31 October 2011). "#55 Roflumilast – COPD relief at last?". CFPCLearn. Archived from the original on 28 March 2023. Retrieved 18 June 2023.
- ↑ Spina D (October 2008). "PDE4 inhibitors: current status". British Journal of Pharmacology. 155 (3): 308–15. doi:10.1038/bjp.2008.307. PMC 2567892. PMID 18660825.
External links
| External sites: | |
|---|---|
| Identifiers: |
|
- Pages using duplicate arguments in template calls
- CS1 maint: date format
- Drugs with non-standard legal status
- Articles with changed InChI identifier
- Chemical articles with unknown parameter in Infobox drug
- Chemical articles without CAS registry number
- Articles without EBI source
- Chemical pages without ChemSpiderID
- Chemical pages without DrugBank identifier
- Articles without KEGG source
- Articles without UNII source
- Drugs missing an ATC code
- Drug has EMA link
- Drugboxes which contain changes to verified fields
- Drugboxes which contain changes to watched fields
- Articles with changed CASNo identifier
- Articles with changed ChemSpider identifier
- Articles with changed EBI identifier
- Benzamides
- Chloropyridines
- Organofluorides
- PDE4 inhibitors
- Phenol ethers
- Cyclopropyl compounds
- AbbVie brands
- AstraZeneca brands
- Takeda Pharmaceutical Company brands
- RTT