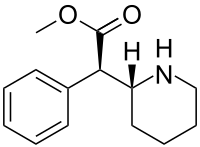
Dexmethylphenidate
 | |
 | |
| Names | |
|---|---|
| Trade names | Focalin, Focalin XR, Attenade, others |
| Other names | d-threo-methylphenidate (D-TMP) |
| |
| Clinical data | |
| Dependence risk | Physical: None Psychological: High |
| Pregnancy category |
|
| Routes of use | By mouth |
| Defined daily dose | 15 mg[2] |
| External links | |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a603014 |
| Legal | |
| License data | |
| Legal status |
|
| Pharmacokinetics | |
| Bioavailability | 11–52% |
| Protein binding | 30% |
| Metabolism | Liver |
| Elimination half-life | 4 hours |
| Excretion | Kidney |
| Chemical and physical data | |
| Formula | C14H19NO2 |
| Molar mass | 233.311 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Dexmethylphenidate, sold under the brand name Focalin among others, is a medication used to treat attention deficit hyperactivity disorder (ADHD) in those over the age of 5 years.[3] If no benefit is seen after 4 weeks it is reasonable to discontinue its use.[3] It is taken by mouth.[3] The immediate release formulation lasts up to 5 hours while the extended release formulation lasts up to 12 hours.[4]
Common side effects include abdominal pain, loss of appetite, and fever.[3] Serious side effects may include abuse, psychosis, sudden cardiac death, mania, anaphylaxis, seizures, and dangerously prolonged erection.[3] Safety during pregnancy and breastfeeding is unclear.[1] Dexmethylphenidate is a central nervous system (CNS) stimulant.[5][3] How it works in ADHD is unclear.[3] It is the more active enantiomer of methylphenidate.[3]
Dexmethylphenidate was approved for medical use in the United States in 2001.[6] It is available as a generic medication.[3] The wholesale cost of a month supply in the United States is about US$8.[7] In 2017, it was the 189th most commonly prescribed medication in the United States, with more than three million prescriptions.[8][9] It is also available in Switzerland.[10]
Medical uses
Dexmethylphenidate is used as a treatment for ADHD, usually along with psychological, educational, behavioral or other forms of treatment. It is proposed that stimulants help ameliorate the symptoms of ADHD by making it easier for the user to concentrate, avoid distraction, and control behavior. Placebo-controlled trials have shown that once-daily dexmethylphenidate XR was effective and generally well tolerated.[5]
Improvements in ADHD symptoms in children were significantly greater for dexmethylphenidate XR versus placebo.[5] It also showed greater efficacy than osmotic controlled-release oral delivery system (OROS) methylphenidate over the first half of the laboratory classroom day but assessments late in the day favoured OROS methylphenidate.[5]
Dosage
The defined daily dose is 15 mg by mouth.[2]
Side effects
Products containing dexmethylphenidate have a side effect profile comparable to those containing methylphenidate.[11]
Mechanism of activity
Methylphenidate is a catecholamine reuptake inhibitor that indirectly increases catecholaminergic neurotransmission by inhibiting the dopamine transporter (DAT) and norepinephrine transporter (NET),[12] which are responsible for clearing catecholamines from the synapse, particularly in the striatum and meso-limbic system.[13] Moreover, it is thought to "increase the release of these monoamines into the extraneuronal space."[14]
Although four stereoisomers of methylphenidate (MPH) are possible, only the threo diastereoisomers are used in modern practice. There is a high eudysmic ratio between the SS and RR enantiomers of MPH. Dexmethylphenidate (d-threo-methylphenidate) is a preparation of the RR enantiomer of methylphenidate.[15][16] In theory, D-TMP (d-threo-methylphenidate) can be anticipated to be twice the strength of the racemic product.[12][17]
| Compd[18] | DAT (Ki) | DA (IC50) | NET (Ki) | NE (IC50) |
|---|---|---|---|---|
| D-TMP | 161 | 23 | 206 | 39 |
| L-TMP | 2250 | 1600 | >10K | 980 |
| DL-TMP | 121 | 20 | 788 | 51 |
Pharmacology
Dexmethylphenidate has a 4–6 hour duration of effect (a long-acting formulation, Focalin XR, which spans 12 hours is also available and has been shown to be as effective as DL (dextro-, levo-)-TMP (threo-methylphenidate) XR (extended release) (Concerta, Ritalin LA), with flexible dosing and good tolerability.[19][20]) It has also been demonstrated to reduce ADHD symptoms in both children[21] and adults.[22] d-MPH has a similar side-effect profile to MPH[11] and can be administered without regard to food intake.[23]
Society and culture
Cost
The wholesale cost of a month supply in the United States is about US$8.[7] In 2017, it was the 189th most commonly prescribed medication in the United States, with more than three million prescriptions.[8][9]
-
Dexmethylphenidate costs (US)
-
Dexmethylphenidate prescriptions (US)
References
- ↑ 1.0 1.1 "Dexmethylphenidate Use During Pregnancy". Drugs.com. Archived from the original on 15 April 2019. Retrieved 15 April 2019.
- ↑ 2.0 2.1 "WHOCC - ATC/DDD Index". www.whocc.no. Archived from the original on 25 January 2021. Retrieved 9 September 2020.
- ↑ 3.0 3.1 3.2 3.3 3.4 3.5 3.6 3.7 3.8 "Dexmethylphenidate Hydrochloride Monograph for Professionals". Drugs.com. American Society of Health-System Pharmacists. Archived from the original on 7 November 2020. Retrieved 15 April 2019.
- ↑ Mosby's Drug Reference for Health Professions - E-Book. Elsevier Health Sciences. 2013. p. 455. ISBN 9780323187602. Archived from the original on 2020-05-15. Retrieved 2019-04-15.
- ↑ 5.0 5.1 5.2 5.3 Moen MD, Keam SJ (December 2009). "Dexmethylphenidate extended release: a review of its use in the treatment of attention-deficit hyperactivity disorder". CNS Drugs. 23 (12): 1057–83. doi:10.2165/11201140-000000000-00000. PMID 19958043.
- ↑ "DailyMed - dexmethylphenidate hydrochloride tablet". dailymed.nlm.nih.gov. Archived from the original on 7 August 2020. Retrieved 15 April 2019.
- ↑ 7.0 7.1 "NADAC as of 2019-02-27". Centers for Medicare and Medicaid Services. Archived from the original on 2019-03-06. Retrieved 3 March 2019.
- ↑ 8.0 8.1 "The Top 300 of 2020". ClinCalc. Archived from the original on 12 February 2021. Retrieved 11 April 2020.
- ↑ 9.0 9.1 "Dexmethylphenidate Hydrochloride - Drug Usage Statistics". ClinCalc. Archived from the original on 12 April 2020. Retrieved 11 April 2020.
- ↑ "Focalin XR". Drugs.com. Archived from the original on 15 April 2019. Retrieved 15 April 2019.
- ↑ 11.0 11.1 Keating GM, Figgitt DP (2002). "Dexmethylphenidate". Drugs. 62 (13): 1899–904, discussion 1905–8. doi:10.2165/00003495-200262130-00009. PMID 12215063.
- ↑ 12.0 12.1 Markowitz JS, Patrick KS (June 2008). "Differential pharmacokinetics and pharmacodynamics of methylphenidate enantiomers: does chirality matter?". Journal of Clinical Psychopharmacology. 28 (3 Suppl 2): S54-61. doi:10.1097/JCP.0b013e3181733560. PMID 18480678.
- ↑ Schweri MM, Skolnick P, Rafferty MF, Rice KC, Janowsky AJ, Paul SM (October 1985). "[3H]Threo-(+/-)-methylphenidate binding to 3,4-dihydroxyphenylethylamine uptake sites in corpus striatum: correlation with the stimulant properties of ritalinic acid esters". Journal of Neurochemistry. 45 (4): 1062–70. doi:10.1111/j.1471-4159.1985.tb05524.x. PMID 4031878.
- ↑ Novartis: FOCALIN XR Overview[permanent dead link] PDF: FOCALIN XR – Full Prescribing Information Archived 2011-07-14 at the Wayback Machine
- ↑ Ding YS, Fowler JS, Volkow ND, Dewey SL, Wang GJ, Logan J, et al. (May 1997). "Chiral drugs: comparison of the pharmacokinetics of [11C]d-threo and L-threo-methylphenidate in the human and baboon brain". Psychopharmacology. 131 (1): 71–8. doi:10.1007/s002130050267. PMID 9181638. Archived from the original on 2019-12-15. Retrieved 2018-08-21.
- ↑ Ding YS, Gatley SJ, Thanos PK, Shea C, Garza V, Xu Y, et al. (September 2004). "Brain kinetics of methylphenidate (Ritalin) enantiomers after oral administration". Synapse. 53 (3): 168–75. CiteSeerX 10.1.1.514.7833. doi:10.1002/syn.20046. PMID 15236349.
- ↑ Davids E, Zhang K, Tarazi FI, Baldessarini RJ (February 2002). "Stereoselective effects of methylphenidate on motor hyperactivity in juvenile rats induced by neonatal 6-hydroxydopamine lesioning". Psychopharmacology. 160 (1): 92–8. doi:10.1007/s00213-001-0962-5. PMID 11862378.
- ↑ Williard RL, Middaugh LD, Zhu HJ, Patrick KS (February 2007). "Methylphenidate and its ethanol transesterification metabolite ethylphenidate: brain disposition, monoamine transporters and motor activity". Behavioural Pharmacology. 18 (1): 39–51. doi:10.1097/FBP.0b013e3280143226. PMID 17218796.
- ↑ McGough JJ, Pataki CS, Suddath R (July 2005). "Dexmethylphenidate extended-release capsules for attention deficit hyperactivity disorder". Expert Review of Neurotherapeutics. 5 (4): 437–41. doi:10.1586/14737175.5.4.437. PMID 16026226.
- ↑ Silva R, Tilker HA, Cecil JT, Kowalik S, Khetani V, Faleck H, Patin J (2004). "Open-label study of dexmethylphenidate hydrochloride in children and adolescents with attention deficit hyperactivity disorder". Journal of Child and Adolescent Psychopharmacology. 14 (4): 555–63. doi:10.1089/cap.2004.14.555. PMID 15662147.
- ↑ Arnold LE, Lindsay RL, Conners CK, Wigal SB, Levine AJ, Johnson DE, et al. (Winter 2004). "A double-blind, placebo-controlled withdrawal trial of dexmethylphenidate hydrochloride in children with attention deficit hyperactivity disorder". Journal of Child and Adolescent Psychopharmacology. 14 (4): 542–54. doi:10.1089/cap.2004.14.542. PMID 15662146.
- ↑ Spencer TJ, Adler LA, McGough JJ, Muniz R, Jiang H, Pestreich L (June 2007). "Efficacy and safety of dexmethylphenidate extended-release capsules in adults with attention-deficit/hyperactivity disorder". Biological Psychiatry. 61 (12): 1380–7. doi:10.1016/j.biopsych.2006.07.032. PMID 17137560.
- ↑ Teo SK, Scheffler MR, Wu A, Stirling DI, Thomas SD, Stypinski D, Khetani VD (February 2004). "A single-dose, two-way crossover, bioequivalence study of dexmethylphenidate HCl with and without food in healthy subjects". Journal of Clinical Pharmacology. 44 (2): 173–8. doi:10.1177/0091270003261899. PMID 14747426.
External links
| External sites: | |
|---|---|
| Identifiers: |
|
- Pages using duplicate arguments in template calls
- All articles with dead external links
- Articles with dead external links from September 2017
- Articles with invalid date parameter in template
- Articles with permanently dead external links
- Webarchive template wayback links
- Chemical articles with unknown parameter in Infobox drug
- Chemical articles without CAS registry number
- Articles without EBI source
- Chemical pages without ChemSpiderID
- Chemical pages without DrugBank identifier
- Articles without KEGG source
- Articles without UNII source
- Drugs missing an ATC code
- Drugboxes which contain changes to verified fields
- Drugboxes which contain changes to watched fields
- Articles with changed CASNo identifier
- RTT
- Enantiopure drugs
- Stimulants
- Piperidines
- Novartis brands
- Norepinephrine-dopamine reuptake inhibitors
- Nootropics
- Methyl esters
- Methylphenidate

