Calcium chloride
 | |
| Names | |
|---|---|
| Other names | Neutral calcium chloride; calcium(II) chloride, calcium dichloride (1:2), E509 |
| |
| External links | |
| AHFS/Drugs.com | Monograph |
| Chemical and physical data | |
| Formula | CaCl2 |
| Molar mass | 110.98 g·mol−1 |
| 3D model (JSmol) | |
| Density |
|
| Melting point | anhydrous[2] 260 °C (500 °F; 533 K) monohydrate, decomposes 175 °C (347 °F; 448 K) dihydrate, decomposes 45.5 °C (113.9 °F; 318.6 K) tetrahydrate, decomposes[2] 30 °C (86 °F; 303 K) hexahydrate, decomposes[1] |
| Boiling point | anhydrous[1] |
| Solubility in water | Anhydrous: 74.5 g/100 mL (20 °C)[3] Hexahydrate: 49.4 g/100 mL (−25 °C) 59.5 g/100 mL (0 °C) 65 g/100 mL (10 °C) 81.1 g/100 mL (25 °C)[1] 102.2 g/100 mL (30.2 °C) α-Tetrahydrate: 90.8 g/100 mL (20 °C) 114.4 g/100 mL (40 °C) Dihydrate: 134.5 g/100 mL (60 °C) 152.4 g/100 mL (100 °C)[4] mg/mL (20 °C) |
| |
| |
Calcium chloride is a salt with the chemical formula CaCl
2. As a medication it is used in cardiac arrest due to high potassium, high magnesium, low calcium, or calcium channel blocker toxicity.[10] It may be given by injection into a vein or bone.[10]
It is a white crystalline solid at room temperature, and it is highly soluble in water.
Other uses include de-icing and dust control. It is also used as a drying agent.[11]
Medical use
Calcium chloride infusions may be used as an intravenous therapy to treat hypocalcemia.
Dosage
It is available as a 10% solution (1.4 mEq per mL).[10]
Other uses
Freezing-point depression

2 for de-icing in Japan
By depressing the freezing point of water, calcium chloride is used to prevent ice formation and is used to de-ice. This application consumes the greatest amount of calcium chloride. Calcium chloride is relatively harmless to plants and soil. As a deicing agent, it is much more effective at lower temperatures than sodium chloride. When distributed for this use, it usually takes the form of small, white spheres a few millimeters in diameter, called prills. Solutions of calcium chloride can prevent freezing at temperatures as low as −52 °C (−62 °F), making it ideal for filling agricultural implement tires as a liquid ballast, aiding traction in cold climates.[12]
Road surfacing

The second largest application of calcium chloride exploits its hygroscopic nature and the tackiness of its hydrates; calcium chloride is highly hygroscopic and its hydration is an exothermic process. A concentrated solution keeps a liquid layer on the surface of dirt roads, which suppresses the formation of dust. It keeps the finer dust particles on the road, providing a cushioning layer. If these are allowed to blow away, the large aggregate begins to shift around and the road breaks down. Using calcium chloride reduces the need for grading by as much as 50% and the need for fill-in materials as much as 80%.[13]
It is also used in domestic and industrial chemical air dehumidifiers.[14]
Food
The average intake of calcium chloride as food additives has been estimated to be 160–345 mg/day.[15] Calcium chloride is permitted as a food additive in the European Union for use as a sequestrant and firming agent with the E number E509. It is considered as generally recognized as safe (GRAS) by the U.S. Food and Drug Administration.[16] Its use in organic crop production is generally prohibited under the US National Organic Program.[17]
As a firming agent, calcium chloride is used in canned vegetables, in firming soybean curds into tofu and in producing a caviar substitute from vegetable or fruit juices.[18] It is commonly used as an electrolyte in sports drinks and other beverages, including bottled water. The extremely salty taste of calcium chloride is used to flavor pickles without increasing the food's sodium content. Calcium chloride's freezing-point depression properties are used to slow the freezing of the caramel in caramel-filled chocolate bars. Also, it is frequently added to sliced apples to maintain texture.
In brewing beer, calcium chloride is sometimes used to correct mineral deficiencies in the brewing water. It affects flavor and chemical reactions during the brewing process, and can also affect yeast function during fermentation.
In cheesemaking, calcium chloride is sometimes added to processed (pasteurized/homogenized) milk to restore the natural balance between calcium and protein in casein. It is added before the coagulant.
Calcium chloride is used to prevent cork spot and bitter pit on apples by spraying on the tree during the late growing season.[19]
Drying tubes are frequently packed with calcium chloride. Kelp is dried with calcium chloride for use in producing sodium carbonate. Anhydrous calcium chloride has been approved by the FDA as a packaging aid to ensure dryness (CPG 7117.02).[20]
The hydrated salt can be dried for re-use but will dissolve in its own water of hydration if heated quickly and form a hard amalgamated solid when cooled.
Metal reduction flux
Similarly, CaCl
2 is used as a flux and electrolyte in the FFC Cambridge electrolysis process for titanium production, where it ensures the proper exchange of calcium and oxygen ions between the electrodes.
Other applications
Calcium chloride is used in concrete mixes to accelerate the initial setting, but chloride ions lead to corrosion of steel rebar, so it should not be used in reinforced concrete.[21] The anhydrous form of calcium chloride may also be used for this purpose and can provide a measure of the moisture in concrete.[22]
Calcium chloride is included as an additive in plastics and in fire extinguishers, in blast furnaces as an additive to control scaffolding (clumping and adhesion of materials that prevent the furnace charge from descending), and in fabric softener as a thinner.
The exothermic dissolution of calcium chloride is used in self-heating cans and heating pads.
In the oil industry, calcium chloride is used to increase the density of solids-free brines. It is also used to provide inhibition of swelling clays in the water phase of invert emulsion drilling fluids.
CaCl
2 acts as flux material, decreasing the melting point, in the Davy process for the industrial production of sodium metal through the electrolysis of molten NaCl.
Calcium chloride is also used in the production of activated charcoal.
Calcium chloride can be used to precipitate fluoride ions from water as insoluble CaF
2.
Calcium chloride is also an ingredient used in ceramic slipware. It suspends clay particles so that they float within the solution, making it easier to use in a variety of slipcasting techniques.
Calcium chloride dihydrate (20 percent by weight) dissolved in ethanol (95 percent ABV) has been used as a sterilant for male animals. The solution is injected into the testes of the animal. Within one month, necrosis of testicular tissue results in sterilization.[23][24]
Cocaine producers in Colombia import tons of calcium chloride to recover solvents that are on the INCB Red List and are more tightly controlled.[25]
Hazards
Although non-toxic in small quantities when wet, the strongly hygroscopic properties of the non-hydrated salt present some hazards. Calcium chloride can act as an irritant by desiccating moist skin. Solid calcium chloride dissolves exothermically, and burns can result in the mouth and esophagus if it is ingested. Ingestion of concentrated solutions or solid products may cause gastrointestinal irritation or ulceration.[26]
Consumption of calcium chloride can lead to hypercalcemia.[27]
Properties

2
Calcium chloride dissolves in water, producing chloride and the aquo complex [Ca(H
2O)
6]2+. In this way, these solutions are sources of "free" calcium and free chloride ions. This description is illustrated by the fact that these solutions react with phosphate sources to give a solid precipitate of calcium phosphate:
- 3 CaCl
2 + 2 PO3−
4 → Ca
3(PO
4)
2 + 6 Cl−
Calcium chloride has a very high enthalpy change of solution, indicated by considerable temperature rise accompanying dissolution of the anhydrous salt in water. This property is the basis for its largest-scale application.
Molten calcium chloride can be electrolysed to give calcium metal and chlorine gas:
- CaCl
2 → Ca + Cl
2
Preparation
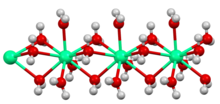
2O)
6]2+ center in crystalline calcium chloride hexahydrate, illustrating the high coordination number typical for calcium complexes.
In much of the world, calcium chloride is derived from limestone as a by-product of the Solvay process, which follows the net reaction below:[11]
- 2 NaCl + CaCO
3 → Na
2CO
3 + CaCl
2
North American consumption in 2002 was 1,529,000 tonnes (3.37 billion pounds).[28] In the US, most of calcium chloride is obtained by purification from brine. As with most bulk commodity salt products, trace amounts of other cations from the alkali metals and alkaline earth metals (groups 1 and 2) and other anions from the halogens (group 17) typically occur.[11]
Occurrence
Calcium chloride occurs as the rare evaporite minerals sinjarite (dihydrate) and antarcticite (hexahydrate).[29][30][31] Another natural hydrate known is ghiaraite – a tetrahydrate.[32][31] The related minerals chlorocalcite (potassium calcium chloride, KCaCl
3) and tachyhydrite (calcium magnesium chloride, CaMg
2Cl
6·12H
2O) are also very rare.[33][34][31] The same is true for rorisite, CaClF (calcium chloride fluoride).[35][31]
See also
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 1.6 1.7 1.8 Lide, David R., ed. (2009). CRC Handbook of Chemistry and Physics (90th ed.). Boca Raton, Florida: CRC Press. ISBN 978-1-4200-9084-0.
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 Pradyot, Patnaik (2019). Handbook of Inorganic Chemicals. The McGraw-Hill Companies, Inc. p. 162. ISBN 978-0-07-049439-8.
- ↑ "Calcium chloride (anhydrous)". ICSC. International Programme on Chemical Safety and the European Commission. Archived from the original on 25 September 2015. Retrieved 24 October 2023.
- ↑ Seidell, Atherton; Linke, William F. (1919). Solubilities of Inorganic and Organic Compounds (2nd ed.). New York: D. Van Nostrand Company. p. 196.
- ↑ 5.0 5.1 5.2 5.3 5.4 5.5 Anatolievich, Kiper Ruslan. "Properties of substance: calcium chloride". chemister.ru. Archived from the original on 24 June 2015. Retrieved 2014-07-07.
- ↑ 6.0 6.1 6.2 6.3 Müller, Ulrich (2006). Inorganic Structural Chemistry (2nd ed.). England: John Wiley & Sons Ltd. p. 33. ISBN 978-0-470-01864-4.
- ↑ 7.0 7.1 7.2 Sigma-Aldrich Co., Calcium chloride.
- ↑ "MSDS of Calcium chloride". fishersci.ca. Fisher Scientific. Archived from the original on 25 September 2015. Retrieved 2014-07-07.
- ↑ Donald E. Garrett (2004). Handbook of Lithium and Natural Calcium Chloride. Elsevier. p. 379. ISBN 978-0080472904. Archived from the original on 31 October 2023. Retrieved 2018-08-29.
Its toxicity upon ingestion, is indicated by the test on rats: oral LD50 (rat) is 1.0–1.4 g/kg (the lethal dose for half of the test animals, in this case rats...)
- ↑ 10.0 10.1 10.2 "Calcium Salts Monograph for Professionals". Drugs.com. Archived from the original on 18 January 2017. Retrieved 29 November 2023.
- ↑ 11.0 11.1 11.2 Robert Kemp, Suzanne E. Keegan "Calcium Chloride" in Ullmann's Encyclopedia of Industrial Chemistry 2000, Wiley-VCH, Weinheim. doi:10.1002/14356007.a04_547
- ↑ "Binary Phase diagram: The Calcium Chloride – water system". Aqueous Solutions Aps. October 2016. Archived from the original on 26 June 2019. Retrieved 2017-04-20.
- ↑ "Dust: Don't Eat It! Control It!". Road Management & Engineering Journal. US Roads (TranSafety Inc.). 1 June 1998. Archived from the original on 29 October 2007. Retrieved 9 August 2006.
- ↑ "Keeping Things Dry". humantouchofchemistry.com. Archived from the original on 26 October 2014. Retrieved 2014-10-23.
- ↑ Calcium Chloride SIDS Initial Assessment Profile, UNEP Publications, SIAM 15, Boston, 22–25 October 2002, pp. 13–14.
- ↑ 21 CFR § 184.1193
- ↑ 7 CFR § 205.602 Archived 29 April 2021 at the Wayback Machine
- ↑ "Apple Caviar Technique". StarChefs Studio. StarChefs.com. April 2004. Archived from the original on 29 June 2022. Retrieved 9 August 2006.
- ↑ "Cork Spot and Bitter Pit of Apples", Richard C. Funt and Michael A. Ellis, Ohioline.osu.edu/factsheet/plpath-fru-01
- ↑ "CPG 7117.02". FDA Compliance Articles. US Food and Drug Administration. March 1995. Archived from the original on 13 December 2007. Retrieved 3 December 2007.
- ↑ "Accelerating Concrete Set Time". Federal Highway Administration. 1 June 1999. Archived from the original on 17 January 2007. Retrieved 16 January 2007.
- ↑ National Research Council (U.S.). Building Research Institute (1962). Adhesives in Building: Selection and Field Application; Pressure-sensitive Tapes. National Academy of Science-National Research Council. pp. 24–5.
- ↑ Koger, Nov 1977, "Calcium Chloride, Practical Necrotizing Agent", Journal of the American Association of Bovine Practitioners (USA), (Nov 1977), v. 12, p. 118–119
- ↑ Jana, K.; Samanta, P.K. (2011). "Clinical evaluation of non-surgical sterilization of male cats with single intra-testicular injection of calcium chloride". BMC Vet. Res. 7: 39. doi:10.1186/1746-6148-7-39. PMC 3152893. PMID 21774835.
- ↑ Smith, Michael; Simpson, Cam (26 October 2020). "Narcos Are Waging a New Drug War Over a Texas Company's Basic Chemical". Bloomberg. Archived from the original on 26 October 2020. Retrieved 26 October 2020.
{{cite web}}: CS1 maint: bot: original URL status unknown (link) - ↑ "Product Safety Assessment (PSA): Calcium Chloride". Dow Chemical Company. 2 May 2006. Archived from the original on 17 September 2009. Retrieved 22 July 2008.
- ↑ "Calcium Chloride Possible Side Affects". www.drugs.com. Archived from the original on 27 July 2020. Retrieved 24 October 2023.
- ↑ Calcium Chloride SIDS Initial Assessment Profile, UNEP Publications, SIAM 15, Boston, 22–25 October 2002, page 11.
- ↑ "Sinjarite". www.mindat.org. Archived from the original on 3 March 2023. Retrieved 24 October 2023.
- ↑ "Antarcticite". www.mindat.org. Archived from the original on 1 May 2023. Retrieved 24 October 2023.
- ↑ 31.0 31.1 31.2 31.3 "List of Minerals". www.ima-mineralogy.org. 21 March 2011. Archived from the original on 15 March 2013. Retrieved 24 October 2023.
- ↑ "Ghiaraite". www.mindat.org. Archived from the original on 3 March 2023. Retrieved 24 October 2023.
- ↑ "Chlorocalcite". www.mindat.org. Archived from the original on 30 May 2023. Retrieved 24 October 2023.
- ↑ "Tachyhydrite". www.mindat.org. Archived from the original on 3 March 2023. Retrieved 24 October 2023.
- ↑ "Rorisite". www.mindat.org. Archived from the original on 3 March 2023. Retrieved 24 October 2023.
- Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 978-0-08-037941-8.
External links
| Identifiers: |
|
|---|
- International Chemical Safety Card 1184[permanent dead link]
- Product and Application Information (Formerly Dow Chemical Calcium Chloride division) Archived 17 September 2023 at the Wayback Machine
- Report on steel corrosion by chloride including CaCl2 Archived 16 June 2011 at the Wayback Machine
- Collection of calcium chloride reports and articles
- Calcium chloride, Anhydrous MSDS
- Difusivity of calcium chloride
- Centers for Disease Control and Prevention Archived 23 March 2018 at the Wayback Machine, National Institutes of Occupational Safety and Health, "Calcium Chloride (anhydrous)"
- Pages using duplicate arguments in template calls
- Webarchive template wayback links
- CS1 maint: bot: original URL status unknown
- Chemical articles with unknown parameter in Infobox drug
- Infobox drug articles with non-default infobox title
- Chemical articles without CAS registry number
- Articles without EBI source
- Chemical pages without ChemSpiderID
- Chemical pages without DrugBank identifier
- Articles without KEGG source
- Articles without UNII source
- Multiple chemicals in Infobox drug
- Multiple chemicals in an infobox that need indexing
- Drugs missing an ATC code
- Drugs with no legal status
- Drugboxes which contain changes to verified fields
- Drugboxes which contain changes to watched fields
- Articles with changed EBI identifier
- All articles with dead external links
- Articles with dead external links from August 2023
- Articles with invalid date parameter in template
- Articles with permanently dead external links
- Portal templates with all redlinked portals
- Calcium compounds
- Chlorides
- Alkaline earth metal halides
- Deliquescent substances
- Desiccants
- Pyrotechnic colorants
- Edible salt
- E-number additives
- Concrete admixtures