Methylprednisolone
 | |
 | |
| Names | |
|---|---|
| Trade names | Medrol, Depo-Medrol (as acetate), Solu-Medrol (as succinate), others |
| Other names | 6α-Methylprednisolone; 11β,17,21-trihydroxy-6α-methyl-δ1-progesterone; 11β,17,21-Trihydroxy-6α-methylpregna-1,4-diene-3,20-dione |
| |
| Clinical data | |
| Drug class | Corticosteroid |
| Main uses | Skin diseases, rheumatic disorders, allergies, asthma, croup, COPD, certain cancers, multiple sclerosis[1] |
| Side effects | Mental health problems, infection, osteoporosis, cataracts, weakness, easy bruising, yeast infections[1] |
| Pregnancy category | |
| Routes of use | By mouth, IM (as acetate), IA (as acetate), IV (as succinate, suleptanate) |
| Defined daily dose | 20 mg (by injection) 7.5 mg (by mouth)[3] |
| External links | |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a682795 |
| Legal | |
| License data | |
| Legal status | |
| Pharmacokinetics | |
| Protein binding | 78% |
| Metabolism | Liver primarily, kidney, tissues; CYP3A4 |
| Elimination half-life | 18–26 hours |
| Excretion | Urine |
| Chemical and physical data | |
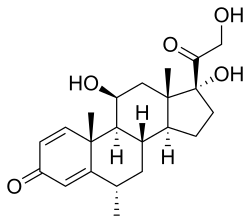
| Formula | C22H30O5 |
| Molar mass | 374.477 g·mol−1 |
| 3D model (JSmol) | |
| Melting point | 228 to 237 °C (442 to 459 °F) |
| |
| |
Methylprednisolone, sold under the brand name Depo-Medrol among others, is a corticosteroid medication used to suppress the immune system and decrease inflammation.[1] Conditions in which it is used include skin diseases, rheumatic disorders, allergies, asthma, croup, COPD, certain cancers, multiple sclerosis, and as add-on therapy for tuberculosis.[1] It is given by mouth, by injection into a vein or muscle, or applied to the skin.[1]
Serious side effects may include mental health problems and an increased risk of infection.[1] Common side effects with long-term use include osteoporosis, cataracts, weakness, easy bruising, and yeast infections.[1] For pregnant women, short-term use around the time of delivery is safe for the baby; however, long-term use during pregnancy may result in harm.[2] Methylprednisolone is in the glucocorticoid family of medication.[1]
Methylprednisolone was approved for medical use in 1955.[4] It is on the World Health Organization's List of Essential Medicines.[5] Methylprednisolone is available as a generic medication.[1] In 2017, it was the 157th most commonly prescribed medication in the United States, with more than four million prescriptions.[6][7]
Medical uses
Like most adrenocortical steroids, methylprednisolone is typically used for its anti-inflammatory effects. However, glucocorticoids have a wide range of effects, including changes to metabolism and immune responses. The list of medical conditions for which methylprednisolone is prescribed is rather long and is similar to other corticosteroids such as prednisolone. Common uses include arthritis therapy and short-term treatment of bronchial inflammation or acute bronchitis due to various respiratory diseases. It is used both in the treatment of acute periods and long-term management of autoimmune diseases, most notably systemic lupus erythematosus. It is also used as a treatment for multiple sclerosis, where it can favour recovery from acute attacks.[8]
Another potential use of methylprednisolone is for vestibular neuritis.[9]
After egg retrieval for a cycle of in vitro fertilization, methylprednisolone may be prescribed to prevent the body from rejecting the embryos being transferred, up to the time of implantation.[10][11]
Dosage
For anaphylaxis the common dose is 125 mg in adults or 1 mg/kg by injection in a child.[12] Doses of 10 mg to 250 mg intravenous or intramuscular are commonly used.[13] Doses of 1 gram intravenous once per day for three days may be used for optic neuritis.[13]
The defined daily dose is 20 mg (parenteral) or 7.5 mg (by mouth).[3]
Side effects
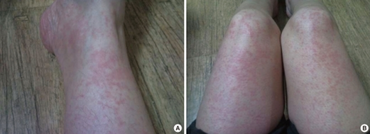
Long-term use of methylprednisolone, as with all corticosteroids, can be associated with hyperglycemia, decreased resistance to infection, swelling of face, weight gain, congestive cardiac insufficiency, fluid and sodium retention, edema, hypertension, increased eye pressure, glaucoma, osteoporosis, and psychosis, especially when used at high doses.[14][15] The most serious side effect occurs after the adrenal glands cease natural production of cortisol, which methylprednisolone will replace. Abrupt cessation of the drug after this occurs can result in a condition known as Addisonian crisis, which can be fatal. To prevent this, the drug is usually prescribed with a tapering dose, including a predosed "dose pack" detailing a specific number of tablets to take at designated times over a several-day period. Pharmacists sometimes advise that this drug may cause sleeplessness and "down" moods.
Measles and chicken pox are very dangerous and potentially fatal for people on methylprednisolone therapy. Exposure to these infections is especially risky for people who are not immune to them. Exposures like these should be reported to a physician immediately, and may be treated with prophylactic immunoglobulin. Also, live, attenuated vaccines can be bad for people taking immunosuppressive doses of methylprednisolone. The exception to this rule is patients receiving complete corticosteroid replacement therapy, e.g., for Addison's disease, who may follow standard immunization protocols.
Pharmacology
Mechanism of action
Unbound glucocorticoids cross cell membranes and bind with high affinity to specific cytoplasmic receptors, modifying transcription and protein synthesis. By this mechanism, glucocorticoids can inhibit leukocyte infiltration at the site of inflammation, interfere with mediators of inflammatory response, and suppress humoral immune responses. The anti-inflammatory actions of corticosteroids are thought to involve phospholipase A2 inhibitory proteins, lipocortins, which control the biosynthesis of potent mediators of inflammation such as prostaglandins and leukotrienes.
Chemistry
Methylprednisolone, or 6α-methylprednisolone, also known as 11β,17,21-trihydroxy-6α-methylpregna-1,4-diene-3,20-dione, is a synthetic pregnane steroid and a derivative of hydrocortisone (11β,17α,21-trihydroxypregn-4-ene-3,20-dione) and prednisolone (11β,17α,21-trihydroxypregn-1,4-diene-3,20-dione).[16][17] A variety of methylprednisolone esters with differing characteristics exist and have been marketed for medical use.[16][17] They include methylprednisolone aceponate (Advantan), methylprednisolone acetate (Depo-Medrol), methylprednisolone succinate (Solu-Medrol), and methylprednisolone suleptanate (Medrosol, Promedrol).[16][17]
Available forms
Methylprednisolone (brand name Medrol) is provided in prepackaged forms for oral use.[15] Methylprednisolone acetate (Depo-Medrol) is a fat-soluble ester of methylprednisolone and is formulated as an aqueous suspension to be administered by intramuscular, intra-articular, soft tissue, or intralesional injection only.[18] It has the potential to cause subcutaneous atrophy in the area administered.[18] Methylprednisolone acetate is not indicated for intravenous use.[18] The only formulation that should be given intravenously is methylprednisolone succinate (Solu-medrol), a water-soluble ester of methylprednisolone.[14]
Society and culture
-
Some 4-mg methylprednisolone tablets by Sandoz
-
Depo-Medrol (methylprednisolone acetate) injectable suspension
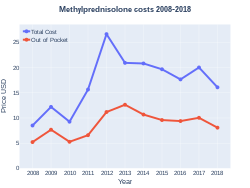
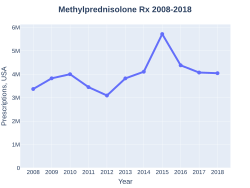
Cost
In 2017, it was the 157th most commonly prescribed medication in the United States, with more than four million prescriptions.[6][7]
-
Methylprednisolone costs (US)
-
Methylprednisolone prescriptions (US)
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 1.6 1.7 1.8 "Methylprednisolone". The American Society of Health-System Pharmacists. Archived from the original on 27 November 2016. Retrieved 8 December 2016.
- ↑ 2.0 2.1 2.2 2.3 "Methylprednisolone Use During Pregnancy". Drugs.com. 20 August 2019. Archived from the original on 22 December 2016. Retrieved 20 February 2020.
- ↑ 3.0 3.1 "WHOCC - ATC/DDD Index". www.whocc.no. Archived from the original on 7 August 2020. Retrieved 17 September 2020.
- ↑ Fischer, Janos; Ganellin, C. Robin (2006). Analogue-based Drug Discovery. John Wiley & Sons. p. 486. ISBN 978-3-527-60749-5. Archived from the original on 2016-12-22.
- ↑ World Health Organization (2019). World Health Organization model list of essential medicines: 21st list 2019. Geneva: World Health Organization. hdl:10665/325771. WHO/MVP/EMP/IAU/2019.06. License: CC BY-NC-SA 3.0 IGO.
- ↑ 6.0 6.1 "The Top 300 of 2020". ClinCalc. Archived from the original on 12 February 2021. Retrieved 11 April 2020.
- ↑ 7.0 7.1 "Methylprednisolone - Drug Usage Statistics". ClinCalc. Archived from the original on 12 April 2020. Retrieved 11 April 2020.
- ↑ Filippini, G.; Brusaferri, F.; Sibley, W. A.; Citterio, A.; Ciucci, G.; Midgard, R.; Candelise, L. (2000). "Corticosteroids or ACTH for acute exacerbations in multiple sclerosis". The Cochrane Database of Systematic Reviews (4): CD001331. doi:10.1002/14651858.CD001331. ISSN 1469-493X. PMID 11034713.
- ↑ Strupp M, Zingler VC, Arbusow V, Niklas D, Maag KP, Dieterich M, Bense S, Theil D, Jahn K, Brandt T (July 2004). "Methylprednisolone, valacyclovir, or the combination for vestibular neuritis". N. Engl. J. Med. 351 (4): 354–61. doi:10.1056/NEJMoa033280. PMID 15269315.
- ↑ "Medications Commonly Used During the IVF Cycle". Continuum Reproductive Center. Archived from the original on 6 September 2013. Retrieved 14 June 2013.
- ↑ "In Vitro Fertilization (IVF)". Reproductive Medicine Associates of Michigan. Archived from the original on 15 May 2013. Retrieved 14 June 2013.
- ↑ Society, Canadian Paediatric. "Emergency treatment of anaphylaxis in infants and children | Canadian Paediatric Society". cps.ca. Archived from the original on 14 October 2022. Retrieved 7 January 2023.
- ↑ 13.0 13.1 Tarascon pocket pharmacopoeia : 2020 deluxe lab-coat edition (21th ed.). Burlington, MA. 2020. ISBN 9781284196160.
- ↑ 14.0 14.1 "Solu-medrol- methylprednisolone sodium succinate injection, powder, for solution Solu-medrol- methylprednisolone sodium succinate kit". DailyMed. 16 December 2019. Archived from the original on 8 August 2020. Retrieved 19 February 2020.
- ↑ 15.0 15.1 "Medrol- methylprednisolone tablet". DailyMed. 9 September 2019. Archived from the original on 8 August 2020. Retrieved 19 February 2020.
- ↑ 16.0 16.1 16.2 J. Elks (14 November 2014). The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. pp. 811–. ISBN 978-1-4757-2085-3. Archived from the original on 8 July 2020. Retrieved 19 June 2020.
- ↑ 17.0 17.1 17.2 Index Nominum 2000: International Drug Directory. Taylor & Francis. 2000. pp. 675–. ISBN 978-3-88763-075-1. Archived from the original on 2020-08-05. Retrieved 2020-06-19.
- ↑ 18.0 18.1 18.2 "Depo-Medrol- methylprednisolone acetate injection, suspension". DailyMed. 18 January 2019. Archived from the original on 21 June 2020. Retrieved 19 February 2020.
External links
| External sites: | |
|---|---|
| Identifiers: |
- Pages using duplicate arguments in template calls
- Chemical articles with unknown parameter in Infobox drug
- Chemical articles without CAS registry number
- Articles without EBI source
- Chemical pages without ChemSpiderID
- Chemical pages without DrugBank identifier
- Articles without KEGG source
- Articles without UNII source
- Drugs missing an ATC code
- Articles with hatnote templates targeting a nonexistent page
- Alcohols
- Chemical substances for emergency medicine
- Glucocorticoids
- Ketones
- Pfizer brands
- Pregnanes
- World Health Organization essential medicines
- RTT