Clobetasol propionate
 | |
| Names | |
|---|---|
| Pronunciation | /kloʊˈbeɪtəsɒl/[1] |
| Trade names | Temovate, Clobex, others |
| |
| Clinical data | |
| Drug class | Corticosteroid[2] |
| Main uses | Eczema, contact dermatitis, seborrheic dermatitis, psoriasis[2] |
| Side effects | Skin irritation, dry skin, redness, pimples, telangiectasia[2] |
| Pregnancy category |
|
| Routes of use | Topical only |
| Defined daily dose | Not established[3] |
| External links | |
| AHFS/Drugs.com | Monograph |
| Legal | |
| License data | |
| Legal status |
|
| Chemical and physical data | |
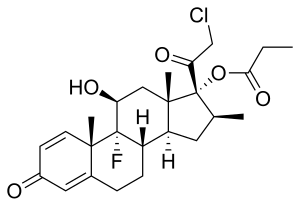
| Formula | C25H32ClFO5 |
| Molar mass | 466.97 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Clobetasol propionate is a corticosteroid used to treat skin conditions such as eczema, contact dermatitis, seborrheic dermatitis, and psoriasis.[2] It is applied to the skin as a cream, ointment, or shampoo.[2][4] Use should be short term and only if other weaker corticosteroids are not effective.[4] Use is not recommended in rosacea or perioral dermatitis.[2]
Common side effects include skin irritation, dry skin, redness, pimples, and telangiectasia.[2] Serious side effects may include adrenal suppression, allergic reactions, cellulitis, and Cushing's syndrome.[2] Use in pregnancy and breastfeeding is of unclear safety.[5] Clobetasol is believed to work by activating steroid receptors.[2] It is a US class I (Europe: class IV) corticosteroid, making it one of the strongest available.[6]
Clobetasol propionate was patented in 1968 and came into medical use in 1978.[7] It is available as a generic medication.[4] A month supply in the United Kingdom costs the NHS about 7.90 £ as of 2019.[4] In the United States the wholesale cost of this amount is about US$66.[8] In 2017, it was the 209th most commonly prescribed medication in the United States, with more than two million prescriptions.[9][10]
Medical uses
Clobetasol propionate is used for the treatment of various skin disorders including eczema, herpes labialis,[11] psoriasis, and lichen sclerosus. It is also used to treat several auto-immune diseases including alopecia areata, lichen planus (auto immune skin nodules), and mycosis fungoides (T-cell skin lymphoma). It is used as first-line treatment for both acute and chronic GVHD of the skin.[12]
Clobetasol propionate is used cosmetically by dark-skinned women for skin whitening, although this use is controversial. The U.S. Food and Drug Administration has not approved it for that purpose, and sales without a prescription are illegal in the U.S. Nonetheless, skin-whitening creams containing this ingredient can sometimes be found in ethnic beauty supply stores in New York City and on the internet. It is also sold internationally, and does not require a prescription in some countries. Whitening creams with clobetasol propionate, such as Hyprogel, can make skin thin and easily bruised, with visible capillaries, and acne. It can also lead to hypertension, elevated blood sugar, suppression of the body's natural steroids, and stretch marks, which may be permanent.[13]
Clobetasol propionate is, along with mercury and hydroquinone, "amongst the most toxic and most used agents in lightening products." Many products sold illegally have higher concentrations of clobetasol propionate than is permitted for prescription drugs.[14]
Dosage
the defined daily dose is not established.[3]
Contraindications
According to the California Environmental Protection Agency, clobetasol propionate should not be used by pregnant women, or women expecting to become pregnant soon, as studies with rats shows a risk of birth defects:[15]
"Studies in the rat following oral administration at dosage levels up to 50 mcg/kg per day revealed that the females exhibited an increase in the number of resorbed embryos and a decrease in the number of living fetuses at the highest dose. Pregnancy: Teratogenic Effects (i.e., possibility of causing abnormalities in fetuses): Pregnancy Category C: Clobetasol propionate has not been tested for teratogenicity when applied topically; however, it is absorbed percutaneously, and when administered subcutaneously it was a significant teratogen in both the rabbit and mouse. Clobetasol propionate has greater teratogenic potential than steroids that are less potent.There are no adequate and well-controlled studies of the teratogenic effects of clobetasol propionate in pregnant women. Temovate Cream and Ointment should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus."
Society and culture
Cost
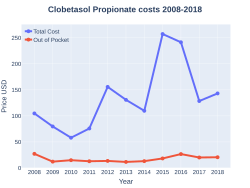
A month supply in the United Kingdom costs the NHS about 7.90 £ as of 2019.[4] In the United States the wholesale cost of this amount is about US$66.[8] In 2017, it was the 209th most commonly prescribed medication in the United States, with more than two million prescriptions.[9][10]
-
ClobetasolPropionate costs (US)
-
ClobetasolPropionate prescriptions (US)
Forms
Clobetasol propionate is marketed and sold worldwide under numerous names including Clobex, Clob-x (Colombia), Clovate, Clobet (Biolab Thailand) Clonovate (T.O. Chemicals, Thailand), Cormax (Watson, US), Haloderm (Switzerland, by ELKO Org), Pentasol (Colombia), Cosvate, Clop (Cadila Healthcare, India), Propysalic (India), Clobex (South Africa), Temovate (US), Dermovate (GlaxoSmithKline, Canada, Estonia, Pakistan, Portugal, Romania, Israel), Olux, ClobaDerm, Tenovate, Dermatovate, Butavate, Movate, Novate, Salac (Argentina), and Powercort, Lotasbat and Kloderma (Indonesia), Lemonvate (Italy), Delor (Ethiopia).
-
Dermovate cream
-
Dermovate cream
References
- ↑ "Clobetasol Propionate Topical Ointment 0.05% Information – Drug Encyclopedia". Kaiser Permanente. Archived from the original on 2021-08-28. Retrieved 2009-10-09.
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 2.6 2.7 2.8 "Clobetasol Propionate Monograph for Professionals". Drugs.com. American Society of Health-System Pharmacists. Archived from the original on 28 August 2021. Retrieved 13 April 2019.
- ↑ 3.0 3.1 "WHOCC - ATC/DDD Index". www.whocc.no. Archived from the original on 1 July 2021. Retrieved 10 September 2020.
- ↑ 4.0 4.1 4.2 4.3 4.4 British national formulary : BNF 76 (76 ed.). Pharmaceutical Press. 2018. p. 1210. ISBN 9780857113382.
- ↑ "Clobetasol topical Use During Pregnancy". Drugs.com. Archived from the original on 28 August 2021. Retrieved 13 April 2019.
- ↑ Ference, JD; Last, AR (15 January 2009). "Choosing topical corticosteroids". American family physician. 79 (2): 135–40. PMID 19178066.
- ↑ Fischer, Jnos; Ganellin, C. Robin (2006). Analogue-based Drug Discovery. John Wiley & Sons. p. 487. ISBN 9783527607495. Archived from the original on 2019-02-28. Retrieved 2019-03-01.
- ↑ 8.0 8.1 "NADAC as of 2019-02-27". Centers for Medicare and Medicaid Services. Archived from the original on 2019-03-06. Retrieved 3 March 2019.
- ↑ 9.0 9.1 "The Top 300 of 2020". ClinCalc. Archived from the original on 12 February 2021. Retrieved 11 April 2020.
- ↑ 10.0 10.1 "Clobetasol Propionate - Drug Usage Statistics". ClinCalc. Archived from the original on 12 April 2020. Retrieved 11 April 2020.
- ↑ Hull C, McKeough M, Sebastian K, Kriesel J, Spruance S (March 2009). "Valacyclovir and topical clobetasol gel for the episodic treatment of herpes labialis: a patient-initiated, double-blind, placebo-controlled pilot trial". Journal of the European Academy of Dermatology and Venereology. 23 (3): 263–7. doi:10.1111/j.1468-3083.2008.03047.x. PMID 19143902.
- ↑ E. Fougera and Co. "CLOBETASOL PROPIONATE CREAM USP, 0.05% CLOBETASOL PROPIONATE OINTMENT USP, 0.05%<". NIH Daily Med. Archived from the original on 2009-05-23. Retrieved 2009-07-03.
- ↑ Saint Louis, Catherine (January 15, 2010). "Creams Offering Lighter Skin May Bring Risks". New York Times. Archived from the original on February 19, 2017. Retrieved February 24, 2017.
- ↑ Gbetoh MH, Amyot M (October 2016). "Mercury, hydroquinone and clobetasol propionate in skin lightening products in West Africa and Canada". Environmental Research. 150: 403–410. doi:10.1016/j.envres.2016.06.030. hdl:1866/19006. PMID 27372064.
- ↑ Office of Environmental Health Hazard Assessment (August 22, 1997). "Chemicals Under Consideration For Possible Listing Via The "Formally Required To Be Labeled Or Identified" Mechanism". California Environmental Protection Agency. Archived from the original on 2001-07-20. Retrieved 2007-05-06.
External links
| Identifiers: |
|
|---|
- "Clobetasol Propionate". Drug Information Portal. U.S. National Library of Medicine. Archived from the original on 2021-08-28. Retrieved 2020-05-16.
- Pages using duplicate arguments in template calls
- Articles with hatnote templates targeting a nonexistent page
- Drugs with non-standard legal status
- Chemical articles with unknown parameter in Infobox drug
- Chemical articles without CAS registry number
- Articles without EBI source
- Chemical pages without ChemSpiderID
- Chemical pages without DrugBank identifier
- Articles without KEGG source
- Articles without UNII source
- Drugs missing an ATC code
- Drugboxes which contain changes to watched fields
- RTT
- Corticosteroid esters
- Corticosteroids
- Propionate esters
- Organochlorides
- Organofluorides