Addison's disease
| Addison's disease | |
|---|---|
| Other names: Addison disease, chronic adrenal insufficiency, hypocortisolism, hypoadrenalism, primary adrenal insufficiency[1] | |
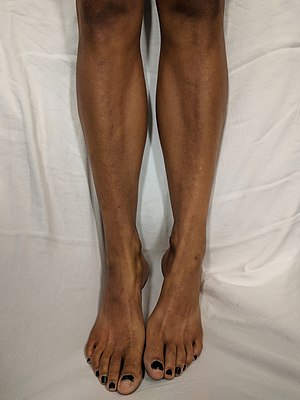 | |
| Darkening of the skin seen on the legs of a white person with Addison's disease | |
| Specialty | Endocrinology |
| Symptoms | Abdominal pain, weakness, weight loss, darkening of the skin[1] |
| Complications | Adrenal crisis[1] |
| Usual onset | Middle-aged females[1] |
| Causes | Problems with the adrenal gland[1] |
| Diagnostic method | Blood tests, urine tests, medical imaging[1] |
| Treatment | Corticosteroid such as hydrocortisone and fludrocortisone[1][2] |
| Frequency | 0.9–1.4 per 10,000 people (developed world)[1][3] |
Addison's disease, also known as primary adrenal insufficiency and hypocortisolism, is a long-term endocrine disorder in which the adrenal glands do not produce enough steroid hormones.[1] Symptoms generally come on slowly and may include abdominal pain, weakness, and weight loss.[1] Darkening of the skin in certain areas may also occur.[4] Under certain circumstances, an adrenal crisis may occur with low blood pressure, vomiting, lower back pain, and loss of consciousness.[1] An adrenal crisis can be triggered by stress, such as from an injury, surgery, or infection.[1]
Addison's disease arises from problems with the adrenal gland such that not enough of the steroid hormone cortisol and possibly aldosterone are produced,[1] most often due to damage by the body's own immune system in the developed world and tuberculosis in the developing world.[5] Other causes include certain medications, sepsis, and bleeding into both adrenal glands.[1][5] Secondary adrenal insufficiency is caused by not enough adrenocorticotropic hormone (ACTH) (produced by the pituitary gland) or corticotropin-releasing hormone (CRH) (produced by the hypothalamus).[1] Despite this distinction, adrenal crises can happen in all forms of adrenal insufficiency.[1] Addison's disease is generally diagnosed by blood tests, urine tests, and medical imaging.[1]
Treatment involves replacing the absent hormones.[1] This involves taking a corticosteroid such as hydrocortisone and fludrocortisone.[1][2] These medications are usually taken by mouth.[1] Lifelong, continuous steroid replacement therapy is required, with regular follow-up treatment and monitoring for other health problems.[6] A high-salt diet may also be useful in some people.[1] If symptoms worsen, an injection of corticosteroid is recommended and people should carry a dose with them.[1] Often, large amounts of intravenous fluids with the sugar dextrose are also required.[1] Without treatment, an adrenal crisis can result in death.[1]
Addison's disease affects about 0.9 to 1.4 per 10,000 people in the developed world.[1][3] It occurs most frequently in middle-aged females.[1] Secondary adrenal insufficiency is more common.[3] Long-term outcomes with treatment are typically good.[7] It is named after Thomas Addison, a graduate of the University of Edinburgh Medical School, who first described the condition in 1855.[8] The adjective "addisonian" is used to describe features of the condition, as well as people with Addison's disease.[9]
Signs and symptoms

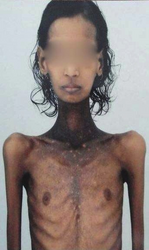
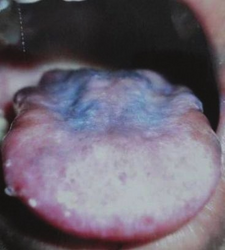
The symptoms of Addison's disease generally develop gradually.[10] Symptoms may include fatigue, muscle weakness, weight loss, nausea, vomiting, loss of appetite, lightheadedness upon standing, irritability, depression, and diarrhea.[10] Some people have cravings for salty foods due to the loss of sodium through their urine.[9][10] Hyperpigmentation of the skin may be seen, particularly when the person lives in a sunny area, as well as darkening of the palmar crease, sites of friction, recent scars, the vermilion border of the lips, and genital skin.[11] These skin changes are not encountered in secondary and tertiary hypoadrenalism.[12]
On physical examination, these clinical signs may be noticed:[9]
- Low blood pressure with or without orthostatic hypotension (blood pressure that decreases with standing)
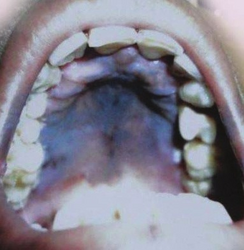
- Darkening (hyperpigmentation) of the skin, including areas not exposed to the sun. Characteristic sites of darkening are skin creases (e.g., of the hands), nipple, and the inside of the cheek (buccal mucosa); also, old scars may darken. This occurs because melanocyte-stimulating hormone (MSH) and ACTH share the same precursor molecule, pro-opiomelanocortin (POMC). After production in the anterior pituitary gland, POMC gets cleaved into gamma-MSH, ACTH, and beta-lipotropin. The subunit ACTH undergoes further cleavage to produce alpha-MSH, the most important MSH for skin pigmentation. In secondary and tertiary forms of adrenal insufficiency, skin darkening does not occur, as ACTH is not overproduced.
Addison's disease is associated with the development of other autoimmune diseases, such as type I diabetes, thyroid disease (Hashimoto's thyroiditis), celiac disease, or vitiligo.[13][14] Addison's disease may be the only manifestation of undiagnosed celiac disease.[13] Both diseases share the same genetic risk factors (HLA-DQ2 and HLA-DQ8 haplotypes).[15]
The presence of Addison's in addition to mucocutaneous candidiasis, hypoparathyroidism, or both, is called autoimmune polyendocrine syndrome type 1.[16] The presence of Addison's in addition to autoimmune thyroid disease, type 1 diabetes, or both, is called autoimmune polyendocrine syndrome type 2.[17]
-
Hyperpigmentation in the neck and chest region
-
Hyperpigmentation in the palate
-
Hyperpigmentation and depapillation of the tongue
-
Acanthosis nigricans in Addison's disease
Adrenal crisis
An "adrenal crisis" or "Addisonian crisis" is a constellation of symptoms that indicates severe adrenal insufficiency. This may be the result of either previously undiagnosed Addison's disease, a disease process suddenly affecting adrenal function (such as adrenal hemorrhage), or an intercurrent problem (e.g., infection, trauma) in someone known to have Addison's disease. It is a medical emergency and potentially life-threatening situation requiring immediate emergency treatment.
Characteristic symptoms are:[10]
- Sudden penetrating pain in the legs, lower back, or abdomen
- Severe vomiting and diarrhea, resulting in dehydration
- Low blood pressure
- Syncope (loss of consciousness and ability to stand)
- Hypoglycemia (reduced level of blood glucose)
- Confusion, psychosis, slurred speech
- Severe lethargy
- Hyponatremia (low sodium level in the blood)
- Hyperkalemia (elevated potassium level in the blood)
- Hypercalcemia (elevated calcium level in the blood)
- Convulsions
- Fever
Causes
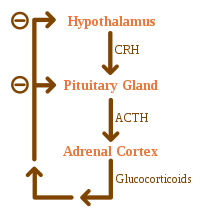
Causes of adrenal insufficiency can be categorized by the mechanism through which they cause the adrenal glands to produce insufficient cortisol. These are adrenal dysgenesis (the gland has not formed adequately during development), impaired steroidogenesis (the gland is present but is biochemically unable to produce cortisol) or adrenal destruction (disease processes leading to glandular damage).[9]
Adrenal destruction
Autoimmune adrenalitis is the most common cause of Addison's disease in the industrialized world. Autoimmune destruction of the adrenal cortex is caused by an immune reaction against the enzyme 21-hydroxylase (a phenomenon first described in 1992).[18] This may be isolated or in the context of autoimmune polyendocrine syndrome (APS type 1 or 2), in which other hormone-producing organs, such as the thyroid and pancreas, may also be affected.[19]
Adrenal destruction is also a feature of adrenoleukodystrophy, and when the adrenal glands are involved in metastasis (seeding of cancer cells from elsewhere in the body, especially lung), hemorrhage (e.g., in Waterhouse–Friderichsen syndrome or antiphospholipid syndrome), particular infections (tuberculosis, histoplasmosis, coccidioidomycosis), or the deposition of abnormal protein in amyloidosis.[20]
Adrenal dysgenesis
All causes in this category are genetic, and generally very rare. These include mutations to the SF1 transcription factor, congenital adrenal hypoplasia due to DAX-1 gene mutations and mutations to the ACTH receptor gene (or related genes, such as in the Triple-A or Allgrove syndrome). DAX-1 mutations may cluster in a syndrome with glycerol kinase deficiency with a number of other symptoms when DAX-1 is deleted together with a number of other genes.[9]
Impaired steroidogenesis
To form cortisol, the adrenal gland requires cholesterol, which is then converted biochemically into steroid hormones. Interruptions in the delivery of cholesterol include Smith–Lemli–Opitz syndrome and abetalipoproteinemia.
Of the synthesis problems, congenital adrenal hyperplasia is the most common (in various forms: 21-hydroxylase, 17α-hydroxylase, 11β-hydroxylase and 3β-hydroxysteroid dehydrogenase), lipoid CAH due to deficiency of StAR and mitochondrial DNA mutations.[9] Some medications interfere with steroid synthesis enzymes (e.g., ketoconazole), while others accelerate the normal breakdown of hormones by the liver (e.g., rifampicin, phenytoin).[9]
Diagnosis
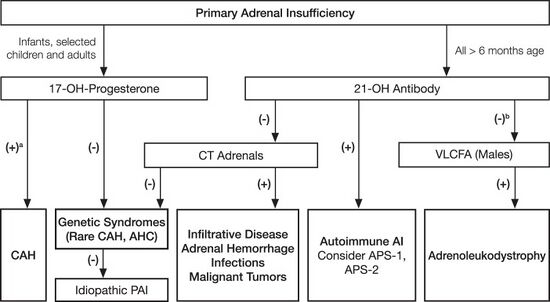
Suggestive features
Routine laboratory investigations may show:[9]
- Low blood sugar (worse in children due to loss of glucocorticoid's glucogenic effects)
- Low blood sodium, due to loss of production of the hormone aldosterone, to the kidney's inability to excrete free water in the absence of sufficient cortisol, and also the effect of corticotropin-releasing hormone to stimulate secretion of ADH.
- High blood potassium, due to loss of production of the hormone aldosterone.
- Eosinophilia and lymphocytosis (increased number of eosinophils or lymphocytes, two types of white blood cells)
- Metabolic acidosis (increased blood acidity), also is due to loss of the hormone aldosterone because sodium reabsorption in the distal tubule is linked with acid/hydrogen ion (H+) secretion. Absent or insufficient levels of aldosterone stimulation of the renal distal tubule leads to sodium wasting in the urine and H+ retention in the serum.
Testing

In suspected cases of Addison's disease, demonstration of low adrenal hormone levels even after appropriate stimulation (called the ACTH stimulation test or synacthen test) with synthetic pituitary ACTH hormone tetracosactide is needed for the diagnosis. Two tests are performed, the short and the long test. Dexamethasone does not cross-react with the assay and can be administered concomitantly during testing.
The short test compares blood cortisol levels before and after 250 micrograms of tetracosactide (intramuscular or intravenous) is given. If, one hour later, plasma cortisol exceeds 170 nmol/l and has risen by at least 330 nmol/l to at least 690 nmol/l, adrenal failure is excluded. If the short test is abnormal, the long test is used to differentiate between primary adrenal insufficiency and secondary adrenocortical insufficiency.
The long test uses 1 mg tetracosactide (intramuscular). Blood is taken 1, 4, 8, and 24 hr later. Normal plasma cortisol level should reach 1000 nmol/l by 4 hr. In primary Addison's disease, the cortisol level is reduced at all stages, whereas in secondary corticoadrenal insufficiency, a delayed but normal response is seen.
Other tests may be performed to distinguish between various causes of hypoadrenalism, including renin and adrenocorticotropic hormone levels, as well as medical imaging - usually in the form of ultrasound, computed tomography or magnetic resonance imaging.
Adrenoleukodystrophy, and the milder form, adrenomyeloneuropathy, cause adrenal insufficiency combined with neurological symptoms. These diseases are estimated to be the cause of adrenal insufficiency in about 35% of diagnosed male with idiopathic Addison's disease, and should be considered in the differential diagnosis of any male with adrenal insufficiency. Diagnosis is made by a blood test to detect very long chain fatty acids.[22]
Treatment
Maintenance
Treatment for Addison's disease involves replacing the missing cortisol, sometimes in the form of hydrocortisone tablets, or prednisone tablets in a dosing regimen that mimics the physiological concentrations of cortisol. Alternatively, one-quarter as much prednisolone may be used for equal glucocorticoid effect as hydrocortisone. Treatment is usually lifelong. In addition, many people require fludrocortisone as replacement for the missing aldosterone.
People with Addison's are often advised to carry information on them (e.g., in the form of a MedicAlert bracelet or information card) for the attention of emergency medical services personnel who might need to attend to their needs.[23][24] It is also recommended that a needle, syringe, and injectable form of cortisol be carried for emergencies.[24] People with Addison's disease are advised to increase their medication during periods of illness or when undergoing surgery or dental treatment.[24] Immediate medical attention is needed when severe infections, vomiting, or diarrhea occur, as these conditions can precipitate an Addisonian crisis. A person who is vomiting may require injections of hydrocortisone instead.[25]
Crisis
Standard therapy involves intravenous injections of glucocorticoids and large volumes of intravenous saline solution with dextrose (glucose). This treatment usually brings rapid improvement. If intravenous access is not immediately available, intramuscular injection of glucocorticoids can be used. When the person can take fluids and medications by mouth, the amount of glucocorticoids is decreased until a maintenance dose is reached. If aldosterone is deficient, maintenance therapy also includes oral doses of fludrocortisone acetate.[26]
Prognosis
Outcomes are typically good when treated. Most can expect to live relatively normal lives. Someone with the disease should be observant of symptoms of an "Addison's crisis" while the body is strained, as in rigorous exercise or being sick, the latter often needing emergency treatment with intravenous injections to treat the crisis.[27]
Individuals with Addison's disease have more than a doubled mortality rate.[28] Furthermore, individuals with Addison's disease and diabetes mellitus have an almost 4 time increase in mortality compared to individuals with only diabetes.[29]
Epidemiology
The frequency rate of Addison's disease in the human population is sometimes estimated at roughly one in 100,000.[30] Some put the number closer to 40–144 cases per million population (1/25,000–1/7,000).[1][31] Addison's can affect persons of any age, sex, or ethnicity, but it typically presents in adults between 30 and 50 years of age.[32] Research has shown no significant predispositions based on ethnicity.[31]
History
Addison's disease is named after Thomas Addison, the British physician who first described the condition in On the Constitutional and Local Effects of Disease of the Suprarenal Capsules (1855).[33] He originally described it as "melasma suprarenale," but later physicians gave it the medical eponym "Addison's disease" in recognition of Addison's discovery.[34]
While the six under Addison in 1855 all had adrenal tuberculosis[35], the term "Addison's disease" does not imply an underlying disease process.
The condition was initially considered a form of anemia associated with the adrenal glands. Because little was known at the time about the adrenal glands (then called "Supra-Renal Capsules"), Addison's monograph describing the condition was an isolated insight. As the adrenal function became better known, Addison's monograph became known as an important medical contribution and a classic example of careful medical observation.[36]
Other animals
Hypoadrenocorticism is uncommon in dogs,[37] and rare in cats, with less than 40 known feline cases worldwide, since first documented in 1983.[38][39] Individual cases have been reported in a grey seal,[40] a red panda,[41] a flying fox,[42] and a sloth.[43]
In dogs, hypoadrenocorticism has been diagnosed in many breeds.[37] Vague symptoms which wax and wane can cause delay in recognition of the presence of the disease.[44] Female dogs appear more affected than male dogs, though this may not be the case in all breeds.[44][45] The disease is most often diagnosed in dogs which are young to middle aged, but it can occur at any age from 4 months to 14 years.[44] Treatment of hypoadrenocorticism must replace the hormones (cortisol and aldosterone) which the dog cannot produce itself.[46] This is achieved either by daily treatment with fludrocortisone, or monthly injections with desoxycorticosterone pivalate (DOCP) and daily treatment with a glucocorticoid, such as prednisone.[46] Several follow-up blood tests are required so that the dose can be adjusted until the dog is receiving the correct amount of treatment, because the medications used in the therapy of hypoadrenocorticism can cause excessive thirst and urination if not prescribed at the lowest effective dose.[46] In anticipation of stressful situations, such as staying in a boarding kennel, dogs require an increased dose of prednisone.[46] Lifelong treatment is required, but the prognosis for dogs with hypoadrenocorticism is very good.[44]
References
- ↑ 1.00 1.01 1.02 1.03 1.04 1.05 1.06 1.07 1.08 1.09 1.10 1.11 1.12 1.13 1.14 1.15 1.16 1.17 1.18 1.19 1.20 1.21 1.22 1.23 1.24 1.25 1.26 "Adrenal Insufficiency and Addison's Disease". National Institute of Diabetes and Digestive and Kidney Diseases. May 2014. Archived from the original on 13 March 2016. Retrieved 13 March 2016.
- ↑ 2.0 2.1 Napier, Catherine; Pearce, Simon H.S. (June 2014). "Current and emerging therapies for Addison's disease". Current Opinion in Endocrinology, Diabetes and Obesity. Philadelphia, Pennsylvania: Lippincott Williams & Wilkins Ltd. 21 (3): 147–53. doi:10.1097/med.0000000000000067. PMID 24755997. Archived from the original on 2019-10-31. Retrieved 2020-08-06.
- ↑ 3.0 3.1 3.2 Brandão Neto, RA; de Carvalho, JF (2014). "Diagnosis and classification of Addison's disease (autoimmune adrenalitis)". Autoimmunity Reviews. 13 (4–5): 408–11. doi:10.1016/j.autrev.2014.01.025. PMID 24424183.
- ↑ James, William D.; Elston, Dirk; Treat, James R.; Rosenbach, Misha A.; Neuhaus, Isaac (2020). "24. Endocrine diseases". Andrews' Diseases of the Skin: Clinical Dermatology (13th ed.). Edinburgh: Elsevier. pp. 497–498. ISBN 978-0-323-54753-6. Archived from the original on 2022-06-08. Retrieved 2022-06-07.
- ↑ 5.0 5.1 Adam, Andy (2014). Grainger & Allison's Diagnostic Radiology (6 ed.). Elsevier Health Sciences. p. 1031. ISBN 9780702061288. Archived from the original on 2016-03-14.
- ↑ Napier, Catherine; Pearce, Simon H.S. (December 2012). "Autoimmune Addison's disease". Presse Médicale. Amsterdam, Netherlands: Elsevier. 41 (12 P 2): e626-35. doi:10.1016/j.lpm.2012.09.010. PMID 23177474.
- ↑ Rajagopalan, Murray Longmore, Ian B. Wilkinson, Supraj R. (2006). Mini Oxford handbook of clinical medicine (6 ed.). Oxford: Oxford University Press. p. 312. ISBN 9780198570714. Archived from the original on 2016-03-14.
- ↑ Rose, Noel R.; Mackay, Ian R. (2014). The autoimmune diseases (5 ed.). San Diego, CA: Elsevier Science. p. 605. ISBN 9780123849304. Archived from the original on 2016-03-14.
- ↑ 9.0 9.1 9.2 9.3 9.4 9.5 9.6 9.7 Ten S, New M, Maclaren N (2001). "Clinical review 130: Addison's disease 2001". The Journal of Clinical Endocrinology and Metabolism. 86 (7): 2909–2922. doi:10.1210/jc.86.7.2909. PMID 11443143. Archived from the original on 2009-08-22. Retrieved 2020-08-06.
- ↑ 10.0 10.1 10.2 10.3 "Addison's Disease". National Endocrine and Metabolic Diseases Information Service. Archived from the original on 28 October 2007. Retrieved 26 October 2007.
- ↑ Freeman, Lynette K.; Chanco Turner, Maria L. (2006). "Addison's disease". Clinics in Dermatology. Amsterdam, Netherlands: Elsevier. 24 (4): 276–280. doi:10.1016/j.clindermatol.2006.04.006. PMID 16828409. Archived from the original on 2020-11-27. Retrieved 2020-08-06.
- ↑ de Herder WW, van der Lely AJ (May 2003). "Addisonian crisis and relative adrenal failure". Reviews in Endocrine and Metabolic Disorders. 4 (2): 143–7. doi:10.1023/A:1022938019091. PMID 12766542.
- ↑ 13.0 13.1 Freeman, Hugh James (2016). "Endocrine manifestations in celiac disease". World Journal of Gastroenterology (Review). Pleasanton, California: Baishideng Publishing Group. 22 (38): 8472–8479. doi:10.3748/wjg.v22.i38.8472. PMC 5064028. PMID 27784959.
- ↑ Zhernakova, Alexandra; Withoff, Sebo; Wijmenga, Cisca (2013). "Clinical implications of shared genetics and pathogenesis in autoimmune diseases". Nature Reviews: Endocrinology (Review). Berlin, Germany: Springer Nature. 9 (11): 646–59. doi:10.1038/nrendo.2013.161. PMID 23959365.
- ↑ Denham, Jolanda M.; Hill, Ivor D. (2013). "Celiac disease and autoimmunity: review and controversies". Current Allergy Asthma Reports (Review). Berlin, Germany: Springer Science+Business Media. 13 (4): 347–53. doi:10.1007/s11882-013-0352-1. PMC 3725235. PMID 23681421.
- ↑ "Autoimmune polyglandular syndrome type 1 | Genetic and Rare Diseases Information Center (GARD) – an NCATS Program". rarediseases.info.nih.gov. Archived from the original on 12 April 2017. Retrieved 26 June 2017.
- ↑ "Autoimmune polyglandular syndrome type 2 | Genetic and Rare Diseases Information Center (GARD) – an NCATS Program". rarediseases.info.nih.gov. Archived from the original on 13 April 2017. Retrieved 26 June 2017.
- ↑ Winqvist O, Karlsson FA, Kämpe O (June 1992). "21-Hydroxylase, a major autoantigen in idiopathic Addison's disease". The Lancet. 339 (8809): 1559–62. doi:10.1016/0140-6736(92)91829-W. PMID 1351548.
- ↑ Husebye, Eystein S.; Perheentupa, J; Rautemaa, R; Kämpe, O (May 2009). "Clinical manifestations and management of patients with autoimmune polyendocrine syndrome type I". Journal of Internal Medicine. 265 (5): 514–29. doi:10.1111/j.1365-2796.2009.02090.x. PMID 19382991.
- ↑ Kennedy, Ron. "Addison's Disease". The Doctors' Medical Library. Archived from the original on 2013-04-12. Retrieved 2013-04-10.
- ↑ Bornstein, Stefan R.; Allolio, Bruno; Arlt, Wiebke; Barthel, Andreas; Don-Wauchope, Andrew; Hammer, Gary D.; Husebye, Eystein S.; Merke, Deborah P.; Murad, M. Hassan; Stratakis, Constantine A.; Torpy, David J. (February 2016). "Diagnosis and Treatment of Primary Adrenal Insufficiency: An Endocrine Society Clinical Practice Guideline". The Journal of Clinical Endocrinology & Metabolism. 101 (2): 364–389. doi:10.1210/jc.2015-1710.
- ↑ Laureti S, Casucci G, Santeusanio F, Angeletti G, Aubourg P, Brunetti P (1996). "X-linked adrenoleukodystrophy is a frequent cause of idiopathic Addison's disease in young adult male patient". The Journal of Clinical Endocrinology and Metabolism. 81 (2): 470–474. doi:10.1210/jc.81.2.470. PMID 8636252.
- ↑ Quinkler M, Dahlqvist P, Husebye ES, Kämpe O (Jan 2015). "A European Emergency Card for adrenal insufficiency can save lives". Eur J Intern Med. 26 (1): 75–6. doi:10.1016/j.ejim.2014.11.006. PMID 25498511.
- ↑ 24.0 24.1 24.2 Michels A, Michels N (1 Apr 2014). "Addison disease: early detection and treatment principles". Am Fam Physician. 89 (7): 563–8. PMID 24695602. Archived from the original on 2015-09-05.
- ↑ White, Katherine (28 July 2004). "What to do in an emergency -Addisonian crisis". Addison's Disease Self Help Group. Archived from the original on 4 March 2016. Retrieved 6 August 2020.
- ↑ "Adrenal Insufficiency and Addison's Disease". National Endocrine and Metabolic Diseases Information Service. Archived from the original on 26 April 2011. Retrieved 26 November 2010.
- ↑ "Addison's disease - Treatment". NHS Choices. Archived from the original on 2016-10-09. Retrieved 2016-10-08.
- ↑ Bergthorsdottir, Ragnhildur; Leonsson-Zachrisson, Maria; Odén, Anders; Johannsson, Gudmundur (2006-12-01). "Premature Mortality in Patients with Addison's Disease: A Population-Based Study". The Journal of Clinical Endocrinology & Metabolism. 91 (12): 4849–4853. doi:10.1210/jc.2006-0076. ISSN 0021-972X. PMID 16968806.
- ↑ Dimitrios Chantzichristos; Anders Persson; Björn Eliasson; Mervete Miftaraj; Stefan Franzén; Ragnhildur Bergthorsdottir; Soffia Gudbjörnsdottir; Ann-Marie Svensson; Gudmundur Johannsson (2016-04-01). Cushing Syndrome and Primary Adrenal Disorders. Meeting Abstracts. Endocrine Society. pp. OR25–4–OR25–4. doi:10.1210/endo-meetings.2016.ahpaa.9.or25-4 (inactive 2020-01-24). Archived from the original on 2019-12-17. Retrieved 2020-08-06.
{{cite book}}: CS1 maint: DOI inactive as of January 2020 (link) - ↑ "Addison Disease". MedicineNet. Archived from the original on 24 June 2007. Retrieved 2007-07-25.
- ↑ 31.0 31.1 Odeke, Sylvester. "Addison Disease". eMedicine. Archived from the original on 7 July 2007. Retrieved 2007-07-25.
- ↑ Volpé, Robert (1990). Autoimmune Diseases of the Endocrine System. CRC Press. p. 299. ISBN 978-0-8493-6849-3.
- ↑ Addison, Thomas (1855). On The Constitutional And Local Effects Of Disease Of The Supra-Renal Capsules. London: Samuel Highley. Archived from the original on 2005-04-14.
- ↑ Physician and Surgeon. Keating & Bryant. 1885. Archived from the original on 2020-11-09. Retrieved 2020-08-06.
- ↑ Patnaik MM, Deshpande AK (May 2008). "Diagnosis–Addison's Disease Secondary to Tuberculosis of the Adrenal Glands". Clinical Medicine & Research. 6 (1): 29. doi:10.3121/cmr.2007.754a. PMC 2442022. PMID 18591375.
- ↑ Bishop PM (1950). "The history of the discovery of Addison's disease". Proceedings of the Royal Society of Medicine. 43 (1): 35–42. doi:10.1177/003591575004300105. PMC 2081266. PMID 15409948.
- ↑ 37.0 37.1 Klein, Susan C.; Peterson, Mark E. (January 2010). "Canine hypoadrenocorticism: Part I". The Canadian Veterinary Journal. 51 (1): 63–9. PMC 2797351. PMID 20357943.
- ↑ 1. Drobatz KJ, Costello MF. Feline Emergency & Critical Care Medicine. Ames, Iowa: Blackwell Publ; 2010. pp. 422–424.
- ↑ Lovelace Tofte, Karen (2018). "Chapter 111. Hypoadrenocorticism". In Norsworthy, Gary D. (ed.). The Feline Patient. John Wiley & Sons. p. 324. ISBN 9781119269038.
- ↑ Stringfield, Cynthia E.; Garne, Michael; Holshuh, H.J. (2000). Addison's disease in a gray seal (Halichoerus grypus). International Association for Aquatic Animal Medicine Proceedings. Archived from the original on 2021-08-27. Retrieved 2020-08-06.
- ↑ Sohn, Pam (10 February 2012). "Endangered red panda dies at Chattanooga Zoo". Times Free Press. Archived from the original on 2018-08-26. Retrieved 2018-08-25.
- ↑ Brock, A. Paige; Hall, Natalie H.; Cooke, Kirsten L.; Reese, David J.; Emerson, Jessica A.; Wellehan, James F.X. Jr (June 2013). "Diagnosis and management of atypical hypoadrenocorticism in a variable flying fox (Pteropus hypomelanus)". Journal of Zoo and Wildlife Medicine. 44 (2): 517–9. doi:10.1638/2012-0276R2.1. PMID 23805580.
- ↑ Kline, Sarah; Rooker, Leah; Nobrega-Lee, Michelle; Guthrie, Amanda (March 2015). "Hypoadrenocorticism (Addison's disease) in a Hoffmann's two-toed sloth (Choloepus hoffmanni)". Journal of Zoo and Wildlife Medicine. 46 (1): 171–174. doi:10.1638/2014-0003R2.1. PMID 25831596.
- ↑ 44.0 44.1 44.2 44.3 Scott-Moncrieff, J. Catharine (2015). "Chapter 12: Hypoadrenocorticism". In Feldman, Edward C.; Nelson, Richard W.; Reusch, Claudia E.; Scott-Moncrieff, J. Catharine R. (eds.). Canine and Feline Endocrinology (4th ed.). Saunders Elsevier. pp. 485–520. ISBN 978-1-4557-4456-5.
- ↑ Boag, Alisdair; Catchpole, Brian (2014). "A Review of the Genetics of Hypoadrenocorticism". Topics in Companion Animal Medicine. 29 (4): 96–101. doi:10.1053/j.tcam.2015.01.001. PMID 25813849.
- ↑ 46.0 46.1 46.2 46.3 Lathan, Patty; Thompson, Ann L. (2018). "Management of hypoadrenocorticism (Addison's disease) in dogs". Veterinary Medicine: Research and Reports. 9: 1–10. doi:10.2147/VMRR.S125617. PMC 6055912. PMID 30050862.
External links
| Classification | |
|---|---|
| External resources |