Cefradine
 | |
| Names | |
|---|---|
| Trade names | Intracef, Velocef |
| |
| Clinical data | |
| WHO AWaRe | |
| Routes of use | Oral, IM, IV |
| External links | |
| AHFS/Drugs.com | International Drug Names |
| MedlinePlus | a601206 |
| Legal | |
| Legal status |
|
| Pharmacokinetics | |
| Bioavailability | Well absorbed |
| Protein binding | <10% |
| Metabolism | Nil |
| Elimination half-life | 0.9 hours |
| Excretion | Kidney, unchanged |
| Chemical and physical data | |
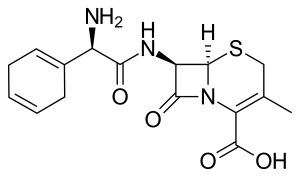
| Formula | C16H19N3O4S |
| Molar mass | 349.41 g·mol−1 |
| 3D model (JSmol) | |
| Melting point | 140 to 142 °C (284 to 288 °F) (dec.) |
| |
| |
Cefradine, also spelled cephradine, is an antibiotic used to treat a wide range of infections.[1] It is generally used in older children and adults.[1] It is available in liquid and capsule form to be taken by mouth.[1] If kidney problems, the dose requires adjusting.[1]
Side effects include allergic reaction, restlessness, stomach upset, blood disorder, liver or kidney problems.[1][2] Used in pregnancy, it is not known to harm the baby.[1] It is a traditional cephalosporin and works by interfering with the bacteria's cell wall.[3] It does not treat MRSA.[3]
In the UK, a typical course of treatment costs the NHS less than £10, as of 2021.[1] it is not available in the United States.[3]
Medical use
- Respiratory tract infections (such as tonsillitis, pharyngitis, and lobar pneumonia) caused by group A beta-hemolytic streptococci and S. pneumoniae (formerly D. pneumonia).[note 1]
- Otitis media caused by group A beta-hemolytic streptococci, S. pneumoniae, H. influenzae, and staphylococci.
- Skin and skin structure infections caused by staphylococci (penicillin-susceptible and penicillin-resistant) and beta-hemolytic streptococci.
- Urinary tract infections, including prostatitis, caused by E. coli, P. mirabilis and Klebsiella species.
Formulations
Cefradine is distributed in the form of capsules containing 250 mg or 500 mg, as a syrup containing 250 mg/5 ml, or in vials for injection containing 500 mg or 1 g.
It is not approved by the FDA for use in the United States.
Synthesis
Birch reduction of D-α-phenylglycine led to diene (2). This was N-protected using tert-butoxycarbonylazide and activated for amide formation via the mixed anhydride method using isobutylchloroformate to give 3. Mixed anhydride 3 reacted readily with 7-aminodesacetoxycephalosporanic acid to give, after deblocking, cephradine (5).

Production names
The antibiotic is produced under many brand names across the world.[7]
- Bangladesh: Ancef, Ancef forte, Aphrin, Avlosef, Cefadin, Cephadin, Cephran, Cephran-DS, Cusef, Cusef DS, Dicef , Dicef forte, Dolocef, Efrad, Extracef, Extracef-DS, Intracef, Kefdrin, Lebac, Lebac Forte, Medicef, Mega-Cef, Megacin, Polycef, Procef, Procef, Procef forte, Rocef, Rocef Forte DS, Sefin, Sefin DS, Sefnin, Sefrad, Sefrad DS, Sefril, Sefril-DS, Sefro, Sefro-HS, Sephar, Sephar-DS, Septa, Sinaceph, SK-Cef, Sk-Cef DS, Supracef and Supracef-F, Torped, Ultrasef, Vecef, Vecef-DS, Velogen, Sinaceph, Velox
- China: Cefradine, Cephradine, Kebili, Saifuding, Shen You, Taididing, Velosef, Xianyi, and Xindadelei
- Colombia: Cefagram, Cefrakov, Cefranil , Cefrex, and Kliacef
- Egypt: Cefadrin, Cefadrine, Cephradine, Cephraforte, Farcosef, Fortecef, Mepadrin, Ultracef, and Velosef
- France: Dexef
- Hong Kong: Cefradine and ChinaQualisef-250
- Indonesia: Dynacef, Velodine, and Velodrom
- Lebanon: Eskacef, Julphacef, and Velosef
- Lithuania: Tafril
- Myanmar: Sinaceph
- Oman: Ceframed, Eskasef, Omadine, and Velocef
- Pakistan: Abidine, Ada-Cef, Ag-cef, Aksosef, Amspor, Anasef, Antimic, Atcosef, Bactocef, Biocef, Biodine, Velora, Velosef
- Peru: Abiocef, Cefradinal, Cefradur, Cefrid, Terbodina II, Velocef, Velomicin
- The Philippines: Altozef, Racep, Senadex, Solphride, Yudinef, Zefadin, Zefradil, and Zolicef
- Poland: Tafril
- Portugal: Cefalmin, Cefradur
- South Africa: Cefril A
- South Korea: Cefradine and Tricef
- Taiwan: Cefadin, Cefamid, Cefin, Cekodin, Cephradine, Ceponin, Lacef, Licef-A, Lisacef, Lofadine, Recef, S-60, Sefree, Sephros, Topcef, Tydine, Unifradine, and U-Save
- UK: Cefradune (Kent)
- Vietnam: Eurosefro and Incef
Cefradine is known as Cefradina in Portuguese and Spanish and is produced by the following companies under this name: AC Farma, Peru; Andromaco, Chile; Anglopharma, Colombia; AZ Pharma, Colombia; Biogalenic, Venezuela; Bussié, Colombia; Elter - Medicamentos Genéricos, Venezuela; Farmindustria, Peru; Genfar, Colombia, Honduras and Peru; La Sante, Peru; La Santé, Colombia; Labesfal, Portugal; Lafrancol, Colombia; LCG, Peru; Marfan, Peru; Memphis, Colombia; Mintlab, Chile; MK, Colombia; Ophalac, Colombia; Procaps, Colombia and Vitalis, Colombia and Peru.
See also
- Cephapirin
- Cephacetrile
- Cefamandole
- Ampicillin (Has the same chemical formula)
Notes
- ↑ Penicillin is the usual drug of choice in the treatment and prevention of streptococcal infections, including the prophylaxis of rheumatic fever. Cefuroxime is generally effective in the eradication of streptococci from the nasopharynx
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 1.6 "5. Infection". British National Formulary (BNF) (82 ed.). London: BMJ Group and the Pharmaceutical Press. September 2021 – March 2022. pp. 559–560. ISBN 978-0-85711-413-6.
{{cite book}}: CS1 maint: date format (link) - ↑ "A - Z Drug List from Drugs.com: Cephradine". Drugs.com. American Society of Health-System Pharmacists. Archived from the original on 18 May 2021. Retrieved 28 November 2021.
- ↑ 3.0 3.1 3.2 Beauduy, Camille E.; Winston, Lisa G. (2020). "43. Beta-lactam and other cell wall - & membrane - active antibiotics". In Katzung, Bertram G.; Trevor, Anthony J. (eds.). Basic and Clinical Pharmacology (15th ed.). New York: McGraw-Hill. pp. 830–832. ISBN 978-1-260-45231-0. Archived from the original on 2021-10-10. Retrieved 2021-11-28.
- ↑ Dolfini JE, Applegate HE, Bach G, Basch H, Bernstein J, Schwartz J, Weisenborn FL (February 1971). "A new class of semisynthetic penicillins and cephalosporins derived from D-2-(1,4-cyclohexadienyl)glycine". Journal of Medicinal Chemistry. 14 (2): 117–9. doi:10.1021/jm00284a008. PMID 5544394.
- ↑ U.S. Patent 3,485,819
- ↑ DE 1931722
- ↑ "Cefradine". Archived from the original on 25 April 2016. Retrieved 5 May 2016.
External links
| Identifiers: |
|
|---|
- Pages using duplicate arguments in template calls
- CS1 maint: date format
- Drugs with non-standard legal status
- Chemical articles with unknown parameter in Infobox drug
- Chemical articles without CAS registry number
- Articles without EBI source
- Chemical pages without ChemSpiderID
- Chemical pages without DrugBank identifier
- Articles without KEGG source
- Articles without UNII source
- Drugs missing an ATC code
- Drugboxes which contain changes to watched fields
- Cephalosporin antibiotics
- Enantiopure drugs