Orlistat
 | |
 | |
| Names | |
|---|---|
| Trade names | Xenical, Alli, others |
| Other names | Tetrahydrolipstatin |
| |
| Clinical data | |
| Drug class | Lipase inhibitor[1] |
| Main uses | Obesity[2] |
| Side effects | Oily stools, loss of bowel control[2] |
| Pregnancy category |
|
| Routes of use | By mouth |
| Typical dose | 120 mg TID[2] |
| External links | |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a601244 |
| Legal | |
| License data | |
| Legal status |
|
| Pharmacokinetics | |
| Bioavailability | Negligible[3] |
| Protein binding | >99% |
| Metabolism | In the GI tract |
| Elimination half-life | 1 to 2 hours |
| Excretion | Fecal |
| Chemical and physical data | |
| Formula | C29H53NO5 |
| Molar mass | 495.745 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Orlistat, sold under the brand name Xenical among others, is a medication used to treat obesity.[2] The effectiveness is modest with people losing about 2–3 kilograms (4.4–6.6 lb) more over the course of a year.[4] It reduces the risk of type II diabetes in people who are obese similar to lifestyle changes.[5] It is taken by mouth.[2]
Common side effects include oily stools and loss of bowel control.[2] Other side effects may include allergic reactions, certain vitamin deficiencies, liver problems, kidney stones, and cholelithiasis.[2] Caution is advised during pregnancy.[1] Its primary works by preventing absorption of fats from the diet.[2] It is used together with diet and exercise.[6]
Orlistat was approved for medical use in Europe in 1998 and the United States in 1999.[2][6] It is available as a generic medication.[1] In Australia, Europe, and the United States it is available without a prescription.[7][2][8] In the United Kingdom 4 weeks of treatment costs about £25.[1] In the United States this amount costs about 80 USD.[9]
Medical uses
Orlistat is used for the treatment of obesity. The amount of weight loss achieved with orlistat varies. In one-year clinical trials, between 35.5% and 54.8% of subjects achieved a 5% or greater decrease in body mass, although not all of this mass was necessarily fat. Between 16.4% and 24.8% achieved at least a 10% decrease in body fat.[10] After orlistat was stopped, a significant number of subjects regained weight—up to 35% of the weight they had lost.[10] It reduces the incidence of diabetes type II in people who are obese around the same amount that lifestyle changes do.[5] Long-term use of orlistat also leads to a very modest reduction in blood pressure (mean reductions of 2.5 and 1.9 mmHg in systolic and diastolic blood pressure respectively).[11]
Dosage
It is taken at a dose of 120 mg three times per day with meals.[2]
Contraindications
Orlistat is contraindicated in:[10]
- Malabsorption
- Hypersensitivity to orlistat
- Reduced gallbladder function (e.g. after cholecystectomy)
- Pregnancy and breastfeeding
- Anorexia and bulimia
- Use caution with: obstructed bile duct, impaired liver function, and pancreatic disease
Side effects
The primary side effects of the drug are gastrointestinal-related, and include steatorrhea (oily, loose stools with excessive flatus due to unabsorbed fats reaching the large intestine), fecal incontinence and frequent or urgent bowel movements.[12] To minimize these effects, foods with high fat content should be avoided; the manufacturer advises consumers to follow a low-fat, reduced-calorie diet. Oily stools and flatulence can be controlled by reducing the dietary fat content to somewhere in the region of 15 grams per meal.[13] The manual for Alli makes it clear that orlistat treatment involves aversion therapy, encouraging the user to associate eating fat with unpleasant treatment effects.[14]
Side effects are most severe when beginning therapy and may decrease in frequency with time;[10] It has also been suggested that the decrease in side effects over time may be associated with long-term compliance with a low-fat diet.[15]
On 26 May 2010, the U.S. Food and Drug Administration (FDA) approved a revised label for Xenical to include new safety information about cases of severe liver injury that have been reported rarely with the use of this medication.[16]
An analysis of over 900 orlistat users in Ontario showed that their rate of acute kidney injury was more than triple that of non-users.[17] The putative mechanism for this effect is postulated to be excessive oxalate absorption from the gut and its subsequent deposition in the kidney, with excessive oxalate absorption being a known consequence of fat malabsorption.
An April 2013 study published in the British Medical Journal[18] looked at 94,695 patients receiving orlistat in the UK between 1999 and 2011. This study showed no evidence of an increased risk of liver injury during treatment. They concluded:
The incidence of acute liver injury was higher in the periods both immediately before and immediately after the start of orlistat treatment. This suggests that the observed increased risks of liver injury linked to the start of treatment may reflect changes in health status associated with the decision to begin treatment rather than any causal effect of the drug.
Long-term
Despite a higher incidence of breast cancer amongst those taking orlistat in early, pooled clinical trial data—the analysis of which delayed FDA review of orlistat[19]—a two-year study published in 1999 found similar rates between orlistat and placebo (0.54% versus 0.51%), and evidence that tumors predated treatment in 3 of the 4 participants who had them.[20] There is evidence from an in vitro study to suggest that the introduction of specific varied preparations containing orlistat, namely the concurrent administration of orlistat and the monoclonal antibody trastuzumab, can induce cell death in breast cancer cells and block their growth.[21]
Fecal fat excretion promotes colon carcinogenesis. In 2006 the results of 30-day study were published indicating that orlistat at a dosage of 200 mg/kg chow administered to rats consuming a high-fat chow and receiving two 25 mg/kg doses of the potent carcinogen 1,2-dimethylhydrazine produced significantly higher numbers of aberrant crypt foci (ACF) colon lesions than did the carcinogen plus high-fat chow without orlistat.[22] ACF lesions are believed to be one of the earliest precursors of colon cancer.[23]
Precautions
Absorption of fat-soluble vitamins and other fat-soluble nutrients is inhibited by the use of orlistat. A multivitamin tablet containing vitamins A,[24] D, E, K, and beta-carotene should be taken once a day, at bedtime, when using orlistat.[10]
Interactions
Orlistat may reduce plasma levels of ciclosporin (also known as "cyclosporin" or "cyclosporine", trade names Sandimmune, Gengraf, Neoral, etc.), an immunosuppressive drug frequently used to prevent transplant rejection; the two drugs should therefore not be administered concomitantly.[10] Orlistat can also impair absorption of the antiarrhythmic amiodarone.[25] The MHRA has recently suggested that Orlistat could theoretically reduce the absorption of antiretroviral HIV medications.[26]
Mechanism of action
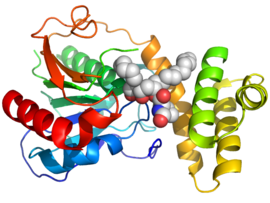
Orlistat works by inhibiting gastric and pancreatic lipases, the enzymes that break down triglycerides in the intestine.[28][29] When lipase activity is blocked, triglycerides from the diet are not hydrolyzed into absorbable free fatty acids, and instead are excreted unchanged. Only trace amounts of orlistat are absorbed systemically; the primary effect is local lipase inhibition within the GI tract after an oral dose. The primary route of elimination is through the feces.
Orlistat was also recently found to inhibit the thioesterase domain of fatty acid synthase (FAS), an enzyme involved in the proliferation of cancer cells but not normal cells. However, potential side effects of Orlistat, such as inhibition of other cellular off-targets or poor bioavailability, might hamper its application as an effective antitumor agent. One profiling study undertook a chemical proteomics approach to look for new cellular targets of orlistat, including its off-targets.[30] Orlistat also shows potential activity against the Trypanosoma brucei parasite.[31]
At the standard prescription dose of 120 mg three times daily, before meals, orlistat prevents approximately 30% of dietary fat from being absorbed.[32] Higher doses do not produce more potent effects.[10]
Chemistry
Orlistat is the saturated derivative of lipstatin, a potent natural inhibitor of pancreatic lipases isolated from the bacterium Streptomyces toxytricini.[33] However, due to its relative simplicity and stability, orlistat was chosen over lipstatin for development as an anti-obesity drug.[34]
History
Society and culture
Cost
At times, such as in spring 2012, orlistat has come into short supply, with consequent price increases because of nonavailability of one of the drug's components.[35]
Legal status

Orlistat has historically been available by prescription only, and this situation continues in Canada. In Australia, the European Union,[8] and the United States, certain formulations of orlistat have been approved for sale without a prescription.
Australia and New Zealand
In Australia and New Zealand, orlistat has been available as a "Pharmacist Only Medicine since 2000.[36] In 2007 the Committee decided to keep orlistat as a Schedule 3 drug, but withdrew its authorization of direct-to-consumer Xenical advertising, stating this "increased pressure on pharmacists to provide orlistat to consumers...this in turn had the potential to result in inappropriate patterns of use".[37]
United States
On 23 January 2006, a U.S. Food and Drug Administration advisory panel voted 11 to 3 to recommend the approval of an OTC formulation of orlistat, to be marketed under the name alli /ˈælaɪ/ by GlaxoSmithKline.[38] Approval was granted on 7 February 2007,[39] and alli became the first weight loss drug officially sanctioned by the U.S. government for over-the-counter use.[40] Consumer advocacy organization Public Citizen opposed over-the-counter approval for orlistat.[41]
Alli became available in the U.S. in June 2007. It is sold as 60 mg capsules—half the dosage of prescription orlistat.[41][40]
Europe
On 21 January 2009, the European Medicines Agency granted approval for the sale of orlistat without a prescription.[8]
At least since September 2017, tablets with 60 mg Orlistat can be freely sold in Swiss drugstores. Formulations with 120 mg per tablet require a prescription, but can be sold without one in pharmacies under an exemption rule, which is based on a list of easily diagnosable diseases.[42]
Generics
U.S. patent protection for Xenical, originally to end on 18 June 2004, was extended by five years (until 2009) by the U.S. Patent and Trademark Office. The extension was granted on 20 July 2002,[43] and expired on 18 June 2009.[44]
Generic orlistat is available in Iran under the brand Venustat manufactured by Aburaihan Pharmaceutical co., in India, under the brands Orlean (Eris), Vyfat, Olistat, Obelit, Orlica and Reeshape.[45] In Russia, orlistat is available under the brand names Xenical (Hoffmann–La Roche), Orsoten/Orsoten Slim (KRKA d. d.) and Xenalten (OBL-Pharm). In Austria, orlistat is available under the brand name Slimox. In Malaysia, orlistat is available under the brand name Cuvarlix and is marketed by Pharmaniaga.
Counterfeit products
In January 2010, the U.S. Food and Drug Administration issued an alert stating that some counterfeit versions of Alli sold over the Internet contain no orlistat, and instead contain the weight-loss drug sibutramine. The concentration of sibutramine in these counterfeit products is at least twice the amount recommended for weight loss.[46]
References
- ↑ 1.0 1.1 1.2 1.3 BNF 81: March-September 2021. BMJ Group and the Pharmaceutical Press. 2021. p. 100. ISBN 978-0857114105.
- ↑ 2.00 2.01 2.02 2.03 2.04 2.05 2.06 2.07 2.08 2.09 2.10 "Orlistat Monograph for Professionals". Drugs.com. Archived from the original on 5 January 2021. Retrieved 9 November 2021.
- ↑ Zhi J, Melia AT, Eggers H, Joly R, Patel IH (1995). "Review of limited systemic absorption of orlistat, a lipase inhibitor, in healthy human volunteers". J Clin Pharmacol. 35 (11): 1103–8. doi:10.1002/j.1552-4604.1995.tb04034.x. PMID 8626884. S2CID 23618845.
- ↑ Padwal R, Li SK, Lau DC (2004). Padwal RS (ed.). "Long-term pharmacotherapy for obesity and overweight". Cochrane Database Syst Rev (3): CD004094. doi:10.1002/14651858.CD004094.pub2. PMC 8078201. PMID 15266516.
- ↑ 5.0 5.1 Gillies, CL; Abrams, KR; Lambert, PC; Cooper, NJ; Sutton, AJ; Hsu, RT; Khunti, K (10 February 2007). "Pharmacological and lifestyle interventions to prevent or delay type 2 diabetes in people with impaired glucose tolerance: systematic review and meta-analysis". BMJ (Clinical Research Ed.). 334 (7588): 299. doi:10.1136/bmj.39063.689375.55. PMC 1796695. PMID 17237299.
- ↑ 6.0 6.1 "Xenical". Archived from the original on 15 April 2021. Retrieved 9 November 2021.
- ↑ "POISONS STANDARD JUNE 2017". Federal Register of Legislation. Therapeutic Goods Administration. June 2017. Archived from the original on 13 December 2020. Retrieved 18 August 2017.
- ↑ 8.0 8.1 8.2 "Chemists to provide obesity pill". BBC News Online. 21 January 2009. Archived from the original on 23 January 2009. Retrieved 22 January 2009.
- ↑ "Compare Alli Prices - GoodRx". GoodRx. Retrieved 9 November 2021.
- ↑ 10.0 10.1 10.2 10.3 10.4 10.5 10.6 "US orlistat label" (PDF). FDA. August 2015. Archived (PDF) from the original on 28 February 2017. Retrieved 18 April 2018. For label updates see FDA index page for NDA 020766 Archived 23 March 2021 at the Wayback Machine
- ↑ Siebenhofer, A; Jeitler, K; Horvath, K; Berghold, A; Posch, N; Meschik, J; Semlitsch, T (2 March 2016). "Long-term effects of weight-reducing drugs in people with hypertension". The Cochrane Database of Systematic Reviews. 3: CD007654. doi:10.1002/14651858.CD007654.pub4. PMID 26934640.
- ↑ "Treating Obesity". NHS. Archived from the original on 13 October 2017. Retrieved 13 July 2013.
- ↑ "FDA Approves alli (orlistat 60 mg capsules) Over-The-Counter" (PDF) (Press release). PR Newswire. 7 February 2007. Archived from the original (PDF) on 24 August 2007. Retrieved 8 April 2007.
- ↑ From page 12 of the Alli Companion Guide, 2007 edition: "They can be an incentive to keep from eating more fat than you really intend to."
- ↑ Mancini MC, Halpern A (2006). "Pharmacological treatment of obesity". Arq Bras Endocrinol Metabol. 50 (2): 377–89. doi:10.1590/S0004-27302006000200024. PMID 16767304. Free full text with registration Archived 12 August 2019 at the Wayback Machine
- ↑ "FDA Drug Safety Communication: Completed safety review of Xenical/Alli (orlistat) and severe liver injury". fda.gov. Archived from the original on 24 April 2019. Retrieved 28 September 2021.
- ↑ "Archived copy". Archived from the original on 31 August 2011. Retrieved 12 April 2011.
{{cite web}}: CS1 maint: archived copy as title (link) - ↑ Douglas, Ian J; Julia Langham; Krishnan Bhaskaran; Ruth Brauer; Liam Smeeth (2013). "Orlistat and the risk of acute liver injury: self controlled case series study in UK Clinical Practice Research Datalink". BMJ. 346: f1936. doi:10.1136/bmj.f1936. PMC 3624963. PMID 23585064.
- ↑ Kolata, Gina (20 January 1999). "Obesity Drug Can Lead to Modest Weight Loss, Study Finds". The New York Times. Archived from the original on 13 August 2019. Retrieved 11 December 2007.
- ↑ Davidson MH, Hauptman J, DiGirolamo M, et al. (1999). "Weight control and risk factor reduction in obese subjects treated for 2 years with orlistat: a randomized controlled trial". JAMA. 281 (3): 235–42. doi:10.1001/jama.281.3.235. PMID 9918478.
- ↑ J. A. Menendez; L. Vellon; R. Lupu (2005). "Antitumoral actions of the anti-obesity drug orlistat (XenicalTM) in breast cancer cells: blockade of cell cycle progression, promotion of apoptotic cell death and PEA3-mediated transcriptional repression of Her2/neu (erbB-2) oncogene". Annals of Oncology. 16 (8): 1253–1267. doi:10.1093/annonc/mdi239. PMID 15870086.
- ↑ Garcia S, da Costa Barros L, Turatti A, Martinello F, Modiano P, Ribeiro-Silva A, de Oliveira Vespúcio M, Uyemura S (2006). "The anti-obesity agent Orlistat is associated to increase in colonic preneoplastic markers in rats treated with a chemical carcinogen". Cancer Lett. 240 (2): 221–4. doi:10.1016/j.canlet.2005.09.011. PMID 16377080.
- ↑ Takayama T, Katsuki S, Takahashi Y, Ohi M, Nojiri S, Sakamaki S, Kato J, Kogawa K, Miyake H, Niitsu Y (1998). "Aberrant crypt foci of the colon as precursors of adenoma and cancer". N Engl J Med. 339 (18): 1277–84. doi:10.1056/NEJM199810293391803. PMID 9791143.
- ↑ "Vitamin A". Archived from the original on 11 March 2015. Retrieved 28 September 2021.
- ↑ Zhi J, Moore R, Kanitra L, Mulligan TE (2003). "Effects of orlistat, a lipase inhibitor, on the pharmacokinetics of three highly lipophilic drugs (amiodarone, fluoxetine, and simvastatin) in healthy volunteers". J Clin Pharmacol. 43 (4): 428–35. doi:10.1177/0091270003252236. PMID 12723464. S2CID 24389189.
- ↑ "Orlistat: theoretical interaction with antiretroviral HIV medicines". MHRA. 13 March 2014. Archived from the original on 29 November 2014. Retrieved 16 November 2014.
- ↑ PDB: 2PX6; Pemble CW, Johnson LC, Kridel SJ, Lowther WT (August 2007). "Crystal structure of the thioesterase domain of human fatty acid synthase inhibited by Orlistat". Nat. Struct. Mol. Biol. 14 (8): 704–9. doi:10.1038/nsmb1265. PMID 17618296. S2CID 2105534.
- ↑ Heck, A. M.; Yanovski, J. A.; Calis, K. A. (March 2000). "Orlistat, a new lipase inhibitor for the management of obesity". Pharmacotherapy. 20 (3): 270–279. doi:10.1592/phco.20.4.270.34882. ISSN 0277-0008. PMC 6145169. PMID 10730683. Archived from the original on 9 June 2020. Retrieved 28 September 2021.
- ↑ Higham, George. "ᐅ Orlistat & Xenical: Do Weight Loss Pills Work? | e-Surgery". e-surgery. Archived from the original on 16 January 2021. Retrieved 9 June 2020.
- ↑ Peng-Yu, Yang (December 2009). "Activity-Based Proteome Profiling of Potential CellularTargets of Orlistat -An FDA-Approved Drug with Anti-TumorActivities". Journal of the American Chemical Society. 132 (2): 656–66. doi:10.1021/ja907716f. PMID 20028024. Archived from the original on 25 October 2021. Retrieved 28 September 2021 – via researchgate.net.
- ↑ Yang, Peng-Yu; Wang, Min; Liu, Kai; Ngai, Mun Hong; Sheriff, Omar; Lear, Martin J.; Sze, Siu Kwan; He, Cynthia Y.; Yao, Shao Q. (2 July 2012). "Parasite-based screening and proteome profiling reveal orlistat, an FDA-approved drug, as a potential anti Trypanosoma brucei agent". Chemistry (Weinheim an der Bergstrasse, Germany). 18 (27): 8403–8413. doi:10.1002/chem.201200482. ISSN 1521-3765. PMID 22674877. Archived from the original on 9 June 2020. Retrieved 28 September 2021.
- ↑ 2006 Physicians' Desk Reference (PDR). Thomson PDR. 2006. ISBN 978-1-56363-527-4.
- ↑ Barbier P, Schneider F (1987). "Syntheses of tetrahydrolipstatin and absolute configuration of tetrahydrolipstatin and lipstatin". Helvetica Chimica Acta. 70 (1): 196–202. doi:10.1002/hlca.19870700124.
- ↑ Pommier A, Pons M, Kocienski P (1995). "The first total synthesis of (−)-lipstatin". Journal of Organic Chemistry. 60 (22): 7334–7339. doi:10.1021/jo00127a045.
- ↑ Jeanne Whalen (20 April 2012). "Glaxo Sells Bulk of Over-the-Counter Drugs". The Wall Street Journal. Archived from the original on 27 August 2016. Retrieved 28 September 2021.
Glaxo said the issue wasn't a lack of interested buyers, but manufacturing problems that have led to shortages of the diet pill and forced the company to delay the product's sale.
- ↑ "Orlistat 120mg capsule blister pack". TGA. 11 April 2000. Retrieved 18 April 2018.
{{cite web}}: CS1 maint: url-status (link) - ↑ "Scheduling of orlistat" (Press release). Australian Therapeutic Goods Administration. 22 February 2007. Archived from the original on 29 December 2007.
- ↑ "Panel Supports Offering Diet Pill Orlistat Over the Counter". The Washington Post. 24 January 2006. pp. A02. Archived from the original on 25 October 2021. Retrieved 10 August 2006.
- ↑ "FDA Approves Orlistat for Over-the-Counter Use" (Press release). U.S. Food and Drug Administration (FDA). 7 February 2007. Archived from the original on 13 May 2009. Retrieved 7 February 2007.
- ↑ 40.0 40.1 Saul, Stephanie (7 February 2007). "Weight-Loss Drug to Be Sold Over the Counter". The New York Times. Archived from the original on 25 October 2021. Retrieved 10 February 2007.
- ↑ 41.0 41.1 Schmid, Randolph E (9 February 2007). "FDA OKs First Nonprescription Diet Pill". USA Today. Archived from the original on 25 October 2021. Retrieved 9 June 2009.
- ↑ http://www.compendium.ch Archived 10 October 2021 at the Wayback Machine, directory of drugs approved in Switzerland
- ↑ Rogan, James E. (30 July 2002). "Certificate Extending Patent Term Under 35 U.S.C. § 156" (PDF). United States Patent and Trademark Office. Archived (PDF) from the original on 29 September 2006. Retrieved 8 April 2007.
- ↑ "Drug Patent Expirations in June 2009". DrugPatentWatch.com, in "Drug Patent Expirations in June 2009". Biotech Blog. 1 June 2009. Archived from the original on 9 June 2009. Retrieved 20 June 2009.
- ↑ Devarajan, Uma (1 March 2009). "Fatty issues". The Deccan Chronicle. Archived from the original on 10 March 2009. Retrieved 26 November 2009.
- ↑ "Fake Alli diet pills can pose health risks". CNN. 23 January 2010. Archived from the original on 25 January 2010. Retrieved 24 January 2010.
Further reading
- Boehm, Marcus F.; McClurg, Michael R.; Pathirana, Charles; Mangelsdorf, David; White, Steven K.; Hebert, Jonathan; Winn, David; Goldman, Mark E.; Heyman, Richard A. (1994). "Synthesis of high specific activity tritium-labeled [3H]-9-cis-retinoic acid and its application for identifying retinoids with unusual binding properties". Journal of Medicinal Chemistry. 37 (3): 408–14. doi:10.1021/jm00029a013. PMID 8308867.
- Hanessian, Stephen; Tehim, Ashok; Chen, Ping (1993). "Total synthesis of (−)-tetrahydrolipstatin". The Journal of Organic Chemistry. 58 (27): 7768. doi:10.1021/jo00079a022.
- Peng-Yu, Yang; Kai, Liu; Mun Hong, Ngai; J.Lear, Martin; R.Wenk, Markus; S.Qin, Yao (2010). "Activity-based proteome profiling of potential cellular targets of Orlistat−an FDA-approved drug with anti-tumor activities". Journal of the American Chemical Society. 132 (2): 656–66. doi:10.1021/ja907716f. PMID 20028024.
- Peng-Yu, Yang; Min, Wang; Kai, Liu; Mun Hong, Ngai; Omar, Sheriff; J.Lear, Martin; Siu Kwan, Sze; Cynthia Y., He; S.Qin, Yao (2012). "Parasite-based screening and proteome profiling reveal orlistat, an FDA-approved drug, as a potential anti Trypanosoma brucei agent". Chemistry: A European Journal. 18 (27): 8403–13. doi:10.1002/chem.201200482. PMID 22674877.
External links
| Identifiers: |
|
|---|
- Pages using duplicate arguments in template calls
- Webarchive template wayback links
- CS1 maint: archived copy as title
- CS1 maint: url-status
- Use dmy dates from November 2020
- Articles with invalid date parameter in template
- Chemical articles with unknown parameter in Infobox drug
- Chemical articles without CAS registry number
- Articles without EBI source
- Chemical pages without ChemSpiderID
- Chemical pages without DrugBank identifier
- Articles without KEGG source
- Articles without UNII source
- Drugs missing an ATC code
- Drug has EMA link
- Drugboxes which contain changes to verified fields
- Drugboxes which contain changes to watched fields
- Articles with hatnote templates targeting a nonexistent page
- Articles with changed DrugBank identifier
- Aldehydes
- Antiobesity drugs
- Carboxylate esters
- Lipase inhibitors
- Hoffmann-La Roche brands
- GlaxoSmithKline brands
- Lactones
- Formamides
- RTT