Fosfomycin
 | |
 | |
| Names | |
|---|---|
| Trade names | Monuril, Monurol, others |
| Other names | Phosphomycin, phosphonomycin, fosfomycin tromethamine |
| |
| Clinical data | |
| Main uses | Bladder infection[1] |
| Pregnancy category |
|
| Routes of use | By mouth |
| Defined daily dose | 3 gm (by mouth)[2] 8 gm (by injection)[2] |
| External links | |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a697008 |
| Legal | |
| Legal status |
|
| Pharmacokinetics | |
| Bioavailability | 30–37% (by mouth, fosfomycin tromethamine); varies with food intake |
| Protein binding | Nil |
| Metabolism | Nil |
| Elimination half-life | 5.7 hours (mean) |
| Excretion | Kidney and fecal, unchanged |
| Chemical and physical data | |
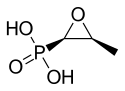
| Formula | C3H7O4P |
| Molar mass | 138.059 g·mol−1 |
| 3D model (JSmol) | |
| Melting point | 94 °C (201 °F) |
| |
| |
Fosfomycin, sold under the brand name Monurol among others, is an antibiotic primarily used to treat bladder infections.[3] It is not recommended for kidney infections.[3] Occasionally it is used for prostate infections.[3] It is generally taken by mouth.[3]
Common side effects include diarrhea, nausea, headache, and vaginal yeast infections.[3] Severe side effects may include anaphylaxis and Clostridioides difficile-associated diarrhea.[3] While use during pregnancy has not been found to be harmful, such use is not recommended.[4] A single dose when breastfeeding appears safe.[4] Fosfomycin works by interfering with the production of the bacterial cell wall.[3]
Fosfomycin was discovered in 1969 and approved for medical use in the United States in 1996.[3][5] It is on the World Health Organization's List of Essential Medicines.[6] It is available as a generic medication.[7] In the United Kingdom it costs the NHS about £5 for a course of treatment.[7] This amount in the United States has a cost of about US$95 as of 2019 while in Canada it costs about 33 CAD as of 2023.[8][9] It was originally produced by certain types of Streptomyces, although it is now made chemically.[5]
Medical uses
Fosfomycin is used to treat bladder infections, where it is usually given as a single dose by mouth.[10] It is lightly less effective at 58% compared to nitrofurantoin at 70%.[11]
It is not recommended for children and those over 75 years old.[12] It is in the 'reserve' group of the WHO AWaRe Classification.[13]
Additional uses have been proposed.[14] The global problem of advancing antimicrobial resistance has led to a renewed interest in its use more recently.[15]
Bacterial sensitivity
The fosfomycin molecule has an epoxide or oxirane ring, which is highly strained and thus very reactive.
Fosfomycin has broad antibacterial activity against both Gram-positive and Gram-negative pathogens, with useful activity against E. faecalis, E. coli, and various Gram-negatives such as Citrobacter and Proteus. Given a greater activity in a low-pH milieu, and predominant excretion in active form into the urine, fosfomycin has found use for the prophylaxis and treatment of UTIs caused by these uropathogens. Of note, activity against S. saprophyticus, Klebsiella, and Enterobacter is variable and should be confirmed by minimum inhibitory concentration testing. Activity against extended-spectrum β-lactamase-producing pathogens, notably ESBL-producing E. coli, is good to excellent, because the drug is not affected by cross-resistance issues. Existing clinical data support use in uncomplicated UTIs, caused by susceptible organisms. However, susceptibility break-points of 64 mg/l should not be applied for systemic infections.
Resistance
Development of bacterial resistance under therapy is a frequent occurrence and makes fosfomycin unsuitable for sustained therapy of severe infections. Mutations that inactivate the nonessential glycerophosphate transporter render bacteria resistant to fosfomycin.[16][17][18]
Enzymes conferring resistance to fosfomycin have also been identified and are encoded both chromosomally and on plasmids.[19]
Three related fosfomycin resistance enzymes (named FosA, FosB, and FosX) are members of the glyoxalase superfamily. These enzymes function by nucleophilic attack on carbon 1 of fosfomycin, which opens the epoxide ring and renders the drug ineffective.
The enzymes differ by the identity of the nucleophile used in the reaction: glutathione for FosA, bacillithiol for FosB,[20][21] and water for FosX.[19]
In general, FosA and FosX enzymes are produced by Gram-negative bacteria, whereas FosB is produced by Gram-positive bacteria.[19]
FosC uses ATP and adds a phosphate group to fosfomycin, thus altering its properties and making the drug ineffective.[22]
Dosage
The defined daily dose is 3 gm by mouth and 8 gm by injection.[2] It is generally used as a single 3 gm dose by mouth for simple bladder infections.[1] The dose does not need to be adjusted in kidney problems.[11]
Side effects
The drug is well tolerated and has a low incidence of harmful side effects.[10] It may be used in pregnancy and during breastfeeding.[1]
Mechanism of action
Fosfomycin is bactericidal and inhibits bacterial cell wall biogenesis by inactivating the enzyme UDP-N-acetylglucosamine-3-enolpyruvyltransferase, also known as MurA.[23] This enzyme catalyzes the committed step in peptidoglycan biosynthesis, namely the ligation of phosphoenolpyruvate (PEP) to the 3'-hydroxyl group of UDP-N-acetylglucosamine. This pyruvate moiety provides the linker that bridges the glycan and peptide portion of peptidoglycan. Fosfomycin is a PEP analog that inhibits MurA by alkylating an active site cysteine residue (Cys 115 in the Escherichia coli enzyme).[24][25]
Fosfomycin enters the bacterial cell through the glycerophosphate transporter.[26]
History
Fosfomycin (originally known as phosphonomycin) was discovered in a joint effort of Merck and Co. and Spain's Compañía Española de Penicilina y Antibióticos (CEPA). It was first isolated by screening broth cultures of Streptomyces fradiae isolated from soil samples for the ability to cause formation of spheroplasts by growing bacteria. The discovery was described in a series of papers published in 1969.[27] CEPA began producing fosfomycin on an industrial scale in 1971 at its Aranjuez facility.[28]
Manufacture
The complete fosfomycin biosynthetic gene cluster from Streptomyces fradiae has been cloned and sequenced and the heterologous production of fosfomycin in S. lividans has been achieved by Ryan Woodyer of the Huimin Zhao and Wilfred van der Donk research groups.[29]
References
- ↑ 1.0 1.1 1.2 "FOSFOMYCIN TROMETAMOL oral - Essential drugs". medicalguidelines.msf.org. Archived from the original on 28 August 2021. Retrieved 30 August 2020.
- ↑ 2.0 2.1 2.2 "WHOCC - ATC/DDD Index". www.whocc.no. Archived from the original on 20 September 2020. Retrieved 30 August 2020.
- ↑ 3.0 3.1 3.2 3.3 3.4 3.5 3.6 3.7 "Fosfomycin Tromethamine Monograph for Professionals". Drugs.com. Archived from the original on 29 October 2019. Retrieved 29 October 2019.
- ↑ 4.0 4.1 "Fosfomycin (Monurol) Use During Pregnancy". Drugs.com. Archived from the original on 29 October 2019. Retrieved 29 October 2019.
- ↑ 5.0 5.1 Finch, Roger G.; Greenwood, David; Whitley, Richard J.; Norrby, S. Ragnar (2010). Antibiotic and Chemotherapy E-Book. Elsevier Health Sciences. p. 259. ISBN 9780702047657. Archived from the original on 2021-08-28. Retrieved 2020-05-25.
- ↑ "World Health Organization model list of essential medicines: 21st list 2019". 2019. hdl:10665/325771.
{{cite journal}}: Cite journal requires|journal=(help) - ↑ 7.0 7.1 British national formulary : BNF 76 (76 ed.). Pharmaceutical Press. 2018. pp. 560–561. ISBN 9780857113382.
- ↑ "Fosfomycin". Archived from the original on 29 October 2019. Retrieved 29 October 2019.
- ↑ Ton, Joey (2 April 2023). "#337 Clear, not cloudy: Antibiotic options for uncomplicated urinary tract infections". CFPCLearn. Archived from the original on 1 July 2023. Retrieved 14 June 2023.
- ↑ 10.0 10.1 Patel SS, Balfour JA, Bryson HM (Apr 1997). "Fosfomycin tromethamine. A review of its antibacterial activity, pharmacokinetic properties and therapeutic efficacy as a single-dose oral treatment for acute uncomplicated lower urinary tract infections". Drugs. 53 (4): 637–656. doi:10.2165/00003495-199753040-00007. PMID 9098664.
- ↑ 11.0 11.1 Ton, Joey (27 July 2020). "#270 Burning Evidence for Fosfomycin in Cystitis". CFPCLearn. Archived from the original on 25 March 2023. Retrieved 15 June 2023.
- ↑ "MONURIL SACHETS 3G". Archived from the original on May 28, 2014. Retrieved May 26, 2014.
- ↑ Zanichelli, Veronica; Sharland, Michael; Cappello, Bernadette; Moja, Lorenzo; Getahun, Haileyesus; Pessoa-Silva, Carmem; Sati, Hatim; van Weezenbeek, Catharina; Balkhy, Hanan; Simão, Mariângela; Gandra, Sumanth; Huttner, Benedikt (1 April 2023). "The WHO AWaRe (Access, Watch, Reserve) antibiotic book and prevention of antimicrobial resistance". Bulletin of the World Health Organization. 101 (4): 290–296. doi:10.2471/BLT.22.288614. ISSN 0042-9686. Archived from the original on 7 May 2023. Retrieved 17 November 2023.
- ↑ Falagas ME, Giannopoulou KP, Kokolakis GN, Rafailidis PI (Apr 2008). "Fosfomycin: use beyond urinary tract and gastrointestinal infections". Clinical Infectious Diseases. 46 (7): 1069–77. doi:10.1086/527442. PMID 18444827.
- ↑ Falagas ME, Grammatikos AP, Michalopoulos A (Oct 2008). "Potential of old-generation antibiotics to address current need for new antibiotics". Expert Review of Anti-Infective Therapy. 6 (5): 593–600. doi:10.1586/14787210.6.5.593. PMID 18847400.
- ↑ Kahan FM, Kahan JS, Cassidy PJ, Kropp H (1974). "The mechanism of action of fosfomycin (phosphonomycin)". Annals of the New York Academy of Sciences. 235 (1): 364–86. Bibcode:1974NYASA.235..364K. doi:10.1111/j.1749-6632.1974.tb43277.x. PMID 4605290.
- ↑ Castañeda-García A, Blázquez J, Rodríguez-Rojas A (2013). "Molecular Mechanisms and Clinical Impact of Acquired and Intrinsic Fosfomycin Resistance". Antibiotics. 2 (2): 217–36. doi:10.3390/antibiotics2020217. PMC 4790336. PMID 27029300.
- ↑ 19.0 19.1 19.2 Rigsby RE, Fillgrove KL, Beihoffer LA, Armstrong RN (2005). "Fosfomycin resistance proteins: a nexus of glutathione transferases and epoxide hydrolases in a metalloenzyme superfamily". Gluthione Transferases and Gamma-Glutamyl Transpeptidases. Methods in Enzymology. Vol. 401. pp. 367–379. doi:10.1016/S0076-6879(05)01023-2. ISBN 9780121828066. PMID 16399398.
- ↑ Sharma SV, Jothivasan VK, Newton GL, Upton H, Wakabayashi JI, Kane MG, Roberts AA, Rawat M, La Clair JJ, Hamilton CJ (Jul 2011). "Chemical and Chemoenzymatic syntheses of bacillithiol: a unique low-molecular-weight thiol amongst low G + C Gram-positive bacteria". Angewandte Chemie. 50 (31): 7101–7104. doi:10.1002/anie.201100196. PMID 21751306.
- ↑ Roberts AA, Sharma SV, Strankman AW, Duran SR, Rawat M, Hamilton CJ (Apr 2013). "Mechanistic studies of FosB: a divalent-metal-dependent bacillithiol-S-transferase that mediates fosfomycin resistance in Staphylococcus aureus". The Biochemical Journal. 451 (1): 69–79. doi:10.1042/BJ20121541. PMC 3960972. PMID 23256780.
- ↑ García P, Arca P, Evaristo Suárez J (Jul 1995). "Product of fosC, a gene from Pseudomonas syringae, mediates fosfomycin resistance by using ATP as cosubstrate". Antimicrobial Agents and Chemotherapy. 39 (7): 1569–73. doi:10.1128/aac.39.7.1569. PMC 162783. PMID 7492106.
- ↑ Brown ED, Vivas EI, Walsh CT, Kolter R (Jul 1995). "MurA (MurZ), the enzyme that catalyzes the first committed step in peptidoglycan biosynthesis, is essential in Escherichia coli". Journal of Bacteriology. 177 (14): 4194–7. doi:10.1128/jb.177.14.4194-4197.1995. PMC 177162. PMID 7608103.
- ↑ Zhu, Jin-Yi; Yang, Yan; Han, Huijong; Betzi, Stephane; Olesen, Sanne H.; Marsilio, Frank; Schönbrunn, Ernst (2012-04-13). "Functional Consequence of Covalent Reaction of Phosphoenolpyruvate with UDP-N-acetylglucosamine 1-Carboxyvinyltransferase (MurA)". Journal of Biological Chemistry. 287 (16): 12657–12667. doi:10.1074/jbc.M112.342725. ISSN 0021-9258. PMC 3339971. PMID 22378791.
- ↑ Krekel, Florian; Samland, Anne K.; Macheroux, Peter; Amrhein, Nikolaus; Evans, Jeremy N. S. (October 2000). "Determination of the pKaValue of C115 in MurA (UDP-N-Acetylglucosamine Enolpyruvyltransferase) fromEnterobacter cloacae†". Biochemistry. 39 (41): 12671–12677. doi:10.1021/bi001310x. ISSN 0006-2960. PMID 11027147.
- ↑ Santoro A, Cappello AR, Madeo M, Martello E, Iacopetta D, Dolce V (Jul 2011). "Interaction of fosfomycin with the glycerol 3-phosphate transporter of Escherichia coli". Biochimica et Biophysica Acta (BBA) - General Subjects. 1810 (12): 1323–1329. doi:10.1016/j.bbagen.2011.07.006. PMID 21791237.
- ↑ Silver, L.L (2011). "Chapter 2, Rational approaches to antibiotic discovery: pre-genomic directed and phenotypic screening". In Dougherty, T.; Pucci, M.J. (eds.). Antibiotic Discovery and Development. Springer. p. 46. doi:10.1007/978-1-4614-1400-1_2. ISBN 978-1-4614-1400-1.
{{cite book}}: Cite has empty unknown parameter:|lastauthoramp=(help) - ↑ Encros About us: Our history. Archived 2011-09-14 at the Wayback Machine
- ↑ Woodyer RD, Shao Z, Thomas PM, Kelleher NL, Blodgett JA, Metcalf WW, van der Donk WA, Zhao H (Nov 2006). "Heterologous production of fosfomycin and identification of the minimal biosynthetic gene cluster". Chemistry & Biology. 13 (11): 1171–82. doi:10.1016/j.chembiol.2006.09.007. PMID 17113999.
External links
| External sites: | |
|---|---|
| Identifiers: |
|
- Pages using duplicate arguments in template calls
- CS1 errors: missing periodical
- CS1 errors: empty unknown parameters
- Webarchive template wayback links
- Articles with hatnote templates targeting a nonexistent page
- Chemical articles with unknown parameter in Infobox drug
- Chemical articles without CAS registry number
- Articles without EBI source
- Chemical pages without ChemSpiderID
- Chemical pages without DrugBank identifier
- Articles without KEGG source
- Articles without UNII source
- Drugs missing an ATC code
- Drugboxes which contain changes to watched fields
- Antibiotics
- Phosphonic acids
- Epoxides
- AbbVie Inc. brands
- RTT
- WHRTT
- World Health Organization essential medicines