Cefprozil
 | |
| Names | |
|---|---|
| Trade names | Cefzil, Cefproz, others |
| Other names | Cephprozil, cefproxil |
| |
| Clinical data | |
| Drug class | Antibiotic (2nd-generation cephalosporin)[1] |
| Main uses | Middle ear infections, skin infections, strep throat, pneumonia[2] |
| Side effects | Diarrhea, nausea, liver problems, rash, dizziness, vaginal yeast infections[3] |
| WHO AWaRe | |
| Pregnancy category |
|
| Routes of use | By mouth |
| External links | |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a698022 |
| Legal | |
| License data | |
| Legal status |
|
| Pharmacokinetics | |
| Bioavailability | 95% |
| Protein binding | 36% |
| Elimination half-life | 1.3 hours |
| Chemical and physical data | |
| Formula | C18H19N3O5S |
| Molar mass | 389.43 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Cefprozil is an antibiotic used to treat middle ear infections, skin infections, strep throat, and pneumonia.[2] It is no longer recommended for sinusitis.[2] It is taken by mouth.[2]
Common side effects include diarrhea, nausea, liver problems, rash, dizziness, and vaginal yeast infections.[3] Other side effects may include allergic reactions and Clostridioides difficile infection.[3] While there is no evidence of harm in pregnancy, such use has not been well studied.[4] It is in the second-generation cephalosporin family of medications.[1]
Cefprozil was patented in 1983 and approved for medical use in 1991.[5][2] It is available as a generic medication.[6] In the United States 20 pills of 500 mg costs about 24 USD as of 2021.[6]
Medical uses
Spectrum of activity
Currently bacteria like Enterobacter aerogenes, Morganella morganii and Pseudomonas aeruginosa are resistant to cefprozil, while Salmonella enterica serotype Agona and streptococci are susceptible to cefprozil. Some bacteria like Brucella abortus, Moraxella catarrhalis and Streptococcus pneumoniae have developed resistance towards cefprozil in varying degrees. Detailed minimum inhibition concentration information is given by the Cefprozil Susceptibility and Resistance Data sheet.[7]
Dosage
In adults it is typically take at a dose of 250 to 500 mg twice per day.[2]
Side effects
Although there is a widely quoted cross-allergy risk of 10% between cephalosporins and penicillin, an article[8] has shown no increased risk for cross-allergy for cefprozil and several other second-generation or later cephalosporins.
Synthesis
This is not a direct copy of Lednicer book like at first glance, but is sourced from the primary reference material.
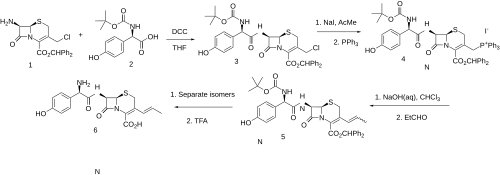
Displacement of the allylic chloride in intermediate (1) with triphenylphosphine gives the phosphonium salt (2). This functionality is then converted to its ylide; condensation with acetaldehyde then leads to the vinyl derivative (3); deprotection then gives cefprozil. Semisynthetic oral cephalosporin consisting of ~90:10 Z/E isomeric mixture.[13]
References
- ↑ 1.0 1.1 Beauduy, Camille E.; Winston, Lisa G. (2020). "43. Beta-lactam and other cell wall - & membrane - active antibiotics". In Katzung, Bertram G.; Trevor, Anthony J. (eds.). Basic and Clinical Pharmacology (15th ed.). New York: McGraw-Hill. p. 832. ISBN 978-1-260-45231-0. Archived from the original on 2021-10-10. Retrieved 2021-11-30.
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 "Cefprozil Monograph for Professionals". Drugs.com. Archived from the original on 8 May 2021. Retrieved 30 December 2021.
- ↑ 3.0 3.1 3.2 "DailyMed - CEFPROZIL tablet, film coated". dailymed.nlm.nih.gov. Archived from the original on 28 December 2021. Retrieved 30 December 2021.
- ↑ "Cefprozil (Cefzil) Use During Pregnancy". Drugs.com. Archived from the original on 23 January 2021. Retrieved 30 December 2021.
- ↑ Fischer J, Ganellin CR (2006). Analogue-based Drug Discovery. John Wiley & Sons. p. 496. ISBN 9783527607495. Archived from the original on 2021-06-19. Retrieved 2021-05-26.
- ↑ 6.0 6.1 "Cefprozil". Archived from the original on 29 October 2016. Retrieved 30 December 2021.
- ↑ "Cefprozil Susceptibility and Resistance Data" (PDF). Archived (PDF) from the original on 19 February 2015. Retrieved 23 July 2013.
- ↑ Pichichero ME (February 2006). "Cephalosporins can be prescribed safely for penicillin-allergic patients" (PDF). The Journal of Family Practice. 55 (2): 106–12. PMID 16451776. Archived from the original on 2012-09-16. Retrieved 2011-02-26.
- ↑ DE 3402642, Hoshi H, et al., issued 1984, assigned to Bristol-Myers
- ↑ US 4520022, Hoshi H, et al., issued 1985, assigned to Bristol-Myers
- ↑ Naito T, Hoshi H, Aburaki S, Abe Y, Okumura J, Tomatsu K, Kawaguchi H (July 1987). "Synthesis and structure-activity relationships of a new oral cephalosporin, BMY-28100 and related compounds". The Journal of Antibiotics. 40 (7): 991–1005. doi:10.7164/antibiotics.40.991. PMID 3624077. Archived from the original on 2018-11-02. Retrieved 2021-05-26.
- ↑ US 4727070, Kaplan MA, et al., issued 1988, assigned to Bristol-Myers
- ↑ TOMATSU, Kozo; Ando, Shigeyuki; Masuyoshi, Shinji; Kondo, Shoichiro; Hirano, Minoru; Miyaki, Takeo; Kawaguchi, Hiroshi (1987). "In vitro and in vivo evaluations of BMY-28100, a new oral cephalosporin". The Journal of Antibiotics. 40 (8): 1175–1183. doi:10.7164/antibiotics.40.1175. ISSN 0021-8820. PMID 3500158.
External links
- Cefprozil Archived 2008-10-01 at the Wayback Machine MedlinePlus Drug Information
| Identifiers: |
|
|---|
- Pages using duplicate arguments in template calls
- Chemical articles with unknown parameter in Infobox drug
- Chemical articles without CAS registry number
- Articles without EBI source
- Chemical pages without ChemSpiderID
- Chemical pages without DrugBank identifier
- Articles without KEGG source
- Articles without UNII source
- Drugs missing an ATC code
- Drugboxes which contain changes to verified fields
- Webarchive template wayback links
- Articles with changed EBI identifier
- Cephalosporin antibiotics
- Phenols
- RTT
- All stub articles
- Antibiotic stubs