Cefoperazone
 | |
 | |
| Names | |
|---|---|
| |
| Clinical data | |
| WHO AWaRe | |
| External links | |
| AHFS/Drugs.com | Micromedex Detailed Consumer Information |
| MedlinePlus | a601206 |
| Pharmacokinetics | |
| Excretion | Hepatic |
| Chemical and physical data | |
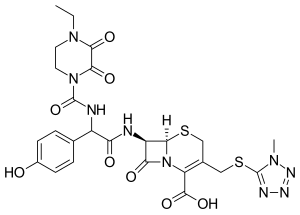
| Formula | C25H27N9O8S2 |
| Molar mass | 645.67 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Cefoperazone is an antibiotic, marketed by Pfizer under the name Cefobid. It is one of few cephalosporin antibiotics effective in treating Pseudomonas bacterial infections which are otherwise resistant to these antibiotics.
It is in the third-generation cephalosporin family of medications and works by interfering with the bacteria's cell wall.[1]
It was patented in 1974 and approved for medical use in 1981.[2] It is no longer available in the United States.[1] Cefoperazone/sulbactam (Sulperazon) is a co-formulation with sulbactam.
Spectrum of bacterial susceptibility
Cefoperazone has a broad spectrum of activity and has been used to target bacteria responsible for causing infections of the respiratory and urinary tract, skin, and the female genital tract. The following represents MIC susceptibility data for a few medically significant microorganisms.
- Haemophilus influenzae: 0.12 - 0.25 µg/ml
- Staphylococcus aureus: 0.125 - 32 µg/ml
- Streptococcus pneumoniae: ≤0.007 - 1 µg/ml[3]
Side effects
Cefoperazone contains an N-methylthiotetrazole (NMTT or 1-MTT) side chain. As the antibiotic is broken down in the body, it releases free NMTT, which can cause hypoprothrombinemia (likely due to inhibition of the enzyme vitamin K epoxide reductase) and a reaction with ethanol similar to that produced by disulfiram (Antabuse), due to inhibition of aldehyde dehydrogenase.[4]
Mechanism of action
Cefoperazone exerts its bactericidal effect by inhibiting the bacterial cell wall synthesis, and sulbactam acts as a beta-lactamase inhibitor, to increase the antibacterial activity of cefoperazone against beta-lactamase-producing organisms.
References
- ↑ 1.0 1.1 Beauduy, Camille E.; Winston, Lisa G. (2020). "43. Beta-lactam and other cell wall - & membrane - active antibiotics". In Katzung, Bertram G.; Trevor, Anthony J. (eds.). Basic and Clinical Pharmacology (15th ed.). New York: McGraw-Hill. p. 832. ISBN 978-1-260-45231-0. Archived from the original on 2021-10-10. Retrieved 2021-11-30.
- ↑ Fischer J, Ganellin CR (2006). Analogue-based Drug Discovery. John Wiley & Sons. p. 494. ISBN 9783527607495. Archived from the original on 2016-12-20. Retrieved 2020-12-19.
- ↑ "Cefoperazone (Cefobid) - The Antimicrobial Index Knowledgebase - TOKU-E". antibiotics.toku-e.com. Archived from the original on 2014-02-01. Retrieved 2020-12-19.
- ↑ Stork CM (2006). "Antibiotics, antifungals, and antivirals". In Nelson LH, Flomenbaum N, Goldfrank LR, Hoffman RL, Howland MD, Lewin NA (eds.). Goldfrank's toxicologic emergencies. New York: McGraw-Hill. p. 847. ISBN 0-07-143763-0. Archived from the original on 2013-06-13. Retrieved 2009-07-03.
External links
| Identifiers: |
|
|---|
- Pages using duplicate arguments in template calls
- Chemical articles with unknown parameter in Infobox drug
- Chemical articles without CAS registry number
- Articles without EBI source
- Chemical pages without ChemSpiderID
- Chemical pages without DrugBank identifier
- Articles without KEGG source
- Articles without UNII source
- Drugs missing an ATC code
- Drugs with no legal status
- Drugboxes which contain changes to watched fields
- Acetaldehyde dehydrogenase inhibitors
- Cephalosporin antibiotics
- Tetrazoles
- Piperazines
- Phenols
- Acetamides
- Lactams
- Pfizer brands