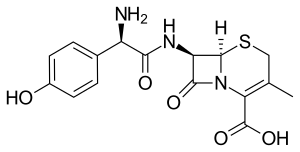
Cefadroxil
 | |
| Names | |
|---|---|
| Trade names | Duricef |
| Other names | Cephadroxil |
| |
| Clinical data | |
| Drug class | Antibiotic (1st generation cephalosporin)[1] |
| Main uses | Skin and soft tissue infection, urinary tract infection[2] |
| Side effects | Indigestion, sore tongue[2] |
| WHO AWaRe | |
| Routes of use | By mouth |
| Typical dose | 500 to 1,000 mg BID[2] |
| External links | |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a682730 |
| Legal | |
| Legal status |
|
| Pharmacokinetics | |
| Protein binding | plasma protein |
| Metabolism | unknown |
| Elimination half-life | 1.5 hours |
| Chemical and physical data | |
| Formula | C16H17N3O5S |
| Molar mass | 363.39 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Cefadroxil, sold under the brand name Duricef, is an antibiotic typically used to treat bacterial infections of the skin and soft tissue and urinary tract.[2][3] It may be used for gram-positive and gram-negativeinfections.[2] It is taken by mouth as a capsule.[2] If kidney problems, the dose may need adjusting.[2]
Common side effects include indigestion and a sore tongue.[2] Less likely is the occurrence of fungal infections, and rarely it may cause joint pains, tiredness, liver problems, difficulty sleeping, and anxiety.[2] Use during pregnancy or breastfeeding does not appear to harm the baby.[2] It is a first-generation cephalosporin.[1] It prevents the last stage of formation of the bacterial cell wall.[4]
Cefadroxil was patented in 1967 and approved for medical use in 1978.[5] It is available as a generic medication.[2] In the United Kingdom, a course of treatment costs the NHS around £20, as of 2021.[2] This amount in the United States is about 10 USD.[6]
Medical use
Cefadroxil is a first-generation cephalosporin antibacterial drug that is the para-hydroxy derivative of cephalexin, and is used similarly in the treatment of mild to moderate susceptible infections such as the bacterium Streptococcus pyogenes, causing the disease popularly called strep throat or streptococcal tonsillitis, urinary tract infection, reproductive tract infection, and skin infections.
Cefadroxil is used as an antibiotic prophylaxis before dental procedures, for patients allergic to penicillins.
Spectrum of activity
Cefadroxil has a broad spectrum of activity and has been effective in treating bacteria responsible for causing tonsillitis, and infections of the skin and urinary tract. The following represents MIC susceptibility data for a few medically significant microorganisms.[7]
- Escherichia coli: 8 μg/ml
- Staphylococcus aureus: 1 – 2 μg/ml
- Streptococcus pneumoniae: ≤1 – >16 μg/ml
Dosage
It is generally used at a dose of 500 to 1,000 mg twice per day.[2]
Cefadroxil is given by mouth, and doses are expressed in terms of the anhydrous substance; 1.04 g of cefadroxil monohydrate is equivalent to about 1 g of anhydrous cefadroxil.
Side effects
The most common side effects of cefadroxil are diarrhea (which, less commonly, may be bloody), nausea, upset stomach, and vomiting. Other side effects include:[3] rashes, hives, and itching.
Pharmacokinetics
Cefadroxil is almost completely absorbed from the gastrointestinal tract. After doses of 500 mg and 1 g by mouth, peak plasma concentrations of about 16 and 30 micrograms/ml, respectively, are obtained after 1.5 to 2.0 hours. Although peak concentrations are similar to those of cefalexin, plasma concentrations are more sustained. Dosage with food does not appear to affect the absorption of cefadroxil. About 20% of cefadroxil is reported to be bound to plasma proteins. Its plasma half-life is about 1.5 hours and is prolonged in patients with renal impairment.
Cefadroxil is widely distributed to body tissues and fluids. It crosses the placenta and appears in breast milk. More than 90% of a dose of cefadroxil may be excreted unchanged in the urine within 24 hours by glomerular filtration and tubular secretion; peak urinary concentrations of 1.8 mg/ml have been reported after a dose of 500 mg. Cefadroxil is removed by haemodialysis.
Veterinary use
It can be used for treating infected wounds on animals. Usually in powder form mixed with water, it has a color and smell similar to Tang. Given orally to animals, the amount is dependent on their weight and severity of infection.
References
- ↑ 1.0 1.1 Beauduy, Camille E.; Winston, Lisa G. (2020). "43. Beta-lactam and other cell wall - & membrane - active antibiotics". In Katzung, Bertram G.; Trevor, Anthony J. (eds.). Basic and Clinical Pharmacology (15th ed.). New York: McGraw-Hill. p. 830. ISBN 978-1-260-45231-0. Archived from the original on 2021-10-10. Retrieved 2021-12-01.
- ↑ 2.00 2.01 2.02 2.03 2.04 2.05 2.06 2.07 2.08 2.09 2.10 2.11 2.12 "5. Infection". British National Formulary (BNF) (82 ed.). London: BMJ Group and the Pharmaceutical Press. September 2021 – March 2022. p. 557. ISBN 978-0-85711-413-6.
{{cite book}}: CS1 maint: date format (link) - ↑ 3.0 3.1 "A - Z Drug List from Drugs.com: Cefadroxil". Drugs.com. American Society of Health-System Pharmacists. Archived from the original on 24 May 2016. Retrieved 20 November 2021.
- ↑ Jain, Mayur S.; Barhate, Shashikant D.; Gayakwad, Bhushan P. (22 March 2018). "Cefadroxil: A Review of Analytical Methods". Asian Journal of Pharmaceutical Analysis. 8 (1): 58–61. doi:10.5958/2231-5675.2018.00011.X. Archived from the original on 2021-04-19. Retrieved 2021-12-01.
- ↑ Fischer J, Ganellin CR (2006). Analogue-based Drug Discovery. John Wiley & Sons. p. 493. ISBN 9783527607495. Archived from the original on 2017-09-10. Retrieved 2020-12-31.
- ↑ "Cefadroxil Prices, Coupons & Savings Tips - GoodRx". GoodRx. Retrieved 30 December 2021.
- ↑ "Cefadroxil, Free Acid Susceptibility and Minimum Inhibitory Concentration (MIC) Data" (PDF). Archived (PDF) from the original on 2016-03-03. Retrieved 2020-11-28.
External links
| Identifiers: |
|
|---|
- Pages using duplicate arguments in template calls
- CS1 maint: date format
- Drugs with non-standard legal status
- Chemical articles with unknown parameter in Infobox drug
- Chemical articles without CAS registry number
- Articles without EBI source
- Chemical pages without ChemSpiderID
- Chemical pages without DrugBank identifier
- Articles without KEGG source
- Articles without UNII source
- Drugs missing an ATC code
- Drugboxes which contain changes to verified fields
- Articles with changed EBI identifier
- Cephalosporin antibiotics
- Phenols
- Acetamides
- RTT