Biochanin A
| |
| |
| Names | |
|---|---|
| IUPAC name
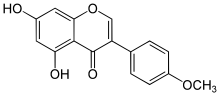
5,7-Dihydroxy-4′-methoxyisoflavone
| |
| Systematic IUPAC name
5,7-Dihydroxy-3-(4-methoxyphenyl)-4H-1-benzopyran-4-one | |
| Other names
Biochanin
4′-Methylgenistein olmelin Biochanine A Biochanin-A Genistein 4-methyl ether | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.007.041 |
| KEGG | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C16H12O5 | |
| Molar mass | 284.267 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Biochanin A is an O-methylated isoflavone. It is a natural organic compound in the class of phytochemicals known as flavonoids. Biochanin A can be found in red clover[1] in soy, in alfalfa sprouts, in peanuts, in chickpea (Cicer arietinum) and in other legumes.
Biochanin A is classified as a phytoestrogen and has putative benefits in dietary cancer prophylaxis.[medical citation needed] It has also been found to be a weak inhibitor of fatty acid amide hydrolase in vitro.[2]
Biochanin A can block the vasoconstriction in a dose-dependent manner due to the inhibition of L-type calcium channels. Such vasodilatory effect, in micromolar concentrations, is of potential clinical interest for the management of cardiovascular pathologies.[3]
Metabolism
The enzyme biochanin-A reductase uses dihydrobiochanin A and NADP+ to produce biochanin A, NADPH, and H+. The enzyme isoflavone-7-O-beta-glucoside 6"-O-malonyltransferase uses malonyl-CoA and biochanin A 7-O-β-D-glucoside to produce CoA and biochanin A 7-O-(6-O-malonyl-β-D-glucoside).
See also
References
- ^ Medjakovic, S.; Jungbauer, A. (2008). "Red clover isoflavones biochanin A and formononetin are potent ligands of the human aryl hydrocarbon receptor". The Journal of Steroid Biochemistry and Molecular Biology. 108 (1–2): 171–177. doi:10.1016/j.jsbmb.2007.10.001. PMID 18060767. S2CID 206495959.
- ^ Thors L, Burston JJ, Alter BJ, McKinney MK, Cravatt BF, Ross RA, Pertwee RG, Gereau RW, Wiley JL, Fowler CJ (2010). "Biochanin A, a naturally occurring inhibitor of fatty acid amide hydrolase". British Journal of Pharmacology. 160 (3): 549–560. doi:10.1111/j.1476-5381.2010.00716.x. PMC 2931556. PMID 20590565.
- ^ Migkos, T., Pourová, J., Vopršalová, M., Auger, C., Schini-Kerth, V., & Mladěnka, P. (2020). "Biochanin A, the Most Potent of 16 Isoflavones, Induces Relaxation of the Coronary Artery Through the Calcium Channel and cGMP-dependent Pathway". Planta medica, 86(10), 708-716. PMID 32408360 doi:10.1055/a-1158-9422
- ECHA InfoCard ID from Wikidata
- Articles containing unverified chemical infoboxes
- Chembox image size set
- Articles with short description
- Short description matches Wikidata
- All articles with unsourced statements
- Articles with unsourced statements from November 2015
- Aromatase inhibitors
- O-methylated isoflavones
- Phytoestrogens
- Selective ERβ agonists