Tamsulosin
 | |
 | |
| Names | |
|---|---|
| Pronunciation | /tæmˈsuːləsən/[1] |
| Trade names | Flomax, others |
| |
| Clinical data | |
| Main uses | Benign prostatic hyperplasia (BPH), chronic prostatitis, kidney stones[2][3][4] |
| Side effects | Dizziness, headache, sleepiness, nausea, blurry vision, sexual problems[5][2] |
| Pregnancy category |
|
| Routes of use | By mouth |
| Defined daily dose | 0.4 mg[6] |
| External links | |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a698012 |
| Legal | |
| License data |
|
| Legal status |
|
| Pharmacokinetics | |
| Bioavailability | 100% (by mouth) |
| Metabolism | Liver |
| Elimination half-life | 9–13 hours |
| Excretion | 76% Kidney |
| Chemical and physical data | |
| Formula | C20H28N2O5S |
| Molar mass | 408.51 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Tamsulosin, sold under the trade name Flomax among others, is a medication used to treat symptomatic benign prostatic hyperplasia (BPH) and chronic prostatitis and to help with the passage of kidney stones.[2][3][4] The evidence for benefit with a kidney stone is better when the stone is larger.[4] It is taken by mouth.[2]
Common side effects include dizziness, headache, sleepiness, nausea, blurry vision, and sexual problems.[2][5] Other side effects may include feeling lightheaded with standing and angioedema.[5] Tamsulosin is an alpha blocker and works by relaxing muscles in the prostate.[7] Specifically it is an α1 adrenergic receptor blocker.[2]
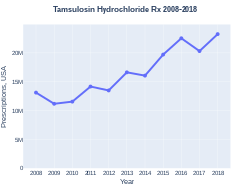
Tamsulosin was approved for medical use in the United States in 1997.[2] It is available as a generic medication and in combination with dutasteride or solifenacin.[5] In the United States, the wholesale cost per dose is less than US$0.10 as of 2018.[8] In the United Kingdom, it costs the NHS 0.04 pounds per dose as of 2021.[5] The modified-release version does not have any particular advantage.[9] In 2017, it was the 35th most commonly prescribed medication in the United States, with more than 20 million prescriptions.[10][11]
Medical uses

Tamsulosin is primarily used for benign prostatic hyperplasia and to help with the passage of kidney stones.[12][13] Tamsulosin, however, appears to be effective only for stones over 4 mm and less than 10 mm in size.[4]
Tamsulosin is also used as an add-on treatment for acute urinary retention. People may void more successfully after catheter removal if they are taking tamsulosin. People taking tamsulosin also are less likely to need re-catheterization.[14]
Tamsulosin does not decrease the overall size of the prostate in men with BPH, and is not recommended for prevention of prostate cancer.[15]
Dosage
The typical dose is 0.4 mg once per day; though this may be increased to 0.8 mg per day.[2] The defined daily dose is 0.4 mg by mouth.[6]
Combination therapy
A 2008 trial found the combination of dutasteride and tamsulosin provides greater symptom benefits compared to either agent alone for treatment of benign prostatic hyperplasia.[16] The CombAT trial became the medication Jalyn. It was approved by the FDA on 14 June 2010.[17] This combination can be useful because it may take up to six months for symptomatic relief to be found when using 5-alpha-reductase inhibitors such as dutasteride compared to alpha-1 receptor blockers, which can provide relief in some cases within 48 hours.[18]
Side effects
- Immunologic: Higher risk of allergic reaction in those with sulfa allergies.[19]
- Eyes: People taking tamsulosin are prone to a complication known as floppy iris syndrome during cataract surgery. Adverse outcomes of the surgery are greatly reduced by the surgeon's prior knowledge of the person's history with this drug, and thus having the option of alternative techniques.[20]
- Severe hypotension.[21][22]
- Persons with cardiac conditions including hypotension, mechanical heart failure (valvular, pulmonary embolism, pericarditis), and congestive heart failure should be monitored carefully while taking tamsulosin.
- Alpha blockers, including prazosin, terazosin, doxazosin, or tamsulosin, do not appear to affect all-cause mortality in heart failure re-hospitalization in those also receiving β-blockers.[23]
- Tamsulosin can also cause retrograde ejaculation, which occurs when semen is redirected to the urinary bladder instead of being ejaculated normally. This is because tamsulosin relaxes the muscles of the urethral sphincters, which are normally closed during ejaculation. This side effect can be mitigated by regular pelvic floor (Kegel) exercise and contracting the pelvic floor during ejaculation.[24]
Mechanism
Tamsulosin is a selective α1 receptor antagonist that has preferential selectivity for the α1A receptor in the prostate versus the α1B receptor in the blood vessels.[25]
When alpha 1 receptors in the bladder neck, the prostate, the ureter, and the urethra are blocked, a relaxation in smooth muscle tissue results.[15] This mechanism decreases resistance to urinary flow, reduces discomfort associated with BPH, and facilitates passage of kidney stones.[15]
Selective action of tamsulosin in alpha 1A/D receptors is controversial and over three quarters of tamsulosin registered human studies are unpublished.[21]
Brand names
Tamsulosin was first marketed in 1996 under the trade name Flomax. The U.S. patent expired in October 2009.[26] The U.S. Food and Drug Administration (FDA) approved generics in March 2010.[27] In 2010, tamsulosin was available as OTC medication in UK.[28]
It is now marketed by various companies under licence, including Boehringer Ingelheim and CSL. Tamsulosin hydrochloride extended-release capsules are marketed under the trade names Urimax 0.4(India), Flomax, Flomaxtra, Contiflo XL, bestflo, Mecir LP (France), Urimax and Pradif,[29] although generic, non-modified-release capsules are still approved and marketed in many countries (such as Canada). Generic extended-release tablets are marketed in most countries of the EEA.[30] In Mexico, it is marketed as Secotex and as Harnal D in Japan and Indonesia and as Harnal OCAS (oral controlled absorption system) in Thailand.[31] In Egypt,[32] Italy, Russia and Iceland, it is marketed under the trade name Omnic by Astellas Pharma Europe. The largest manufacturer of tamsulosin, drug substance, is Synthon BV (The Netherlands). Tamsulosin hydrochloride is marketed in Bangladesh under the trade names Uromax, Prostanil MR, Tamisol MR, Tamsin.[citation needed]
Society and culture
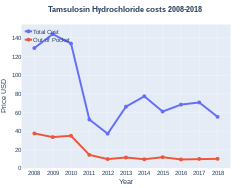
Cost
In 2017, it was the 35th most commonly prescribed medication in the United States, with more than 20 million prescriptions.[10][11] In the United Kingdom, it costs the NHS 0.04 pounds per dose as of 2021.[5]
-
Tamsulosin costs (US)
-
Tamsulosin prescriptions (US)
References
- ↑ "Tamsulosin". Merriam-Webster Dictionary.
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 2.6 2.7 "Tamsulosin Hydrochloride Monograph for Professionals". Drugs.com. AHFS. Archived from the original on 11 October 2018. Retrieved 24 December 2018.
- ↑ 3.0 3.1 "Prostatitis". NHS. 19 October 2017. Archived from the original on 18 April 2019. Retrieved 24 December 2018.
- ↑ 4.0 4.1 4.2 4.3 Wang, RC; Smith-Bindman, R; Whitaker, E; Neilson, J; Allen, IE; Stoller, ML; Fahimi, J (7 September 2016). "Effect of Tamsulosin on Stone Passage for Ureteral Stones: A Systematic Review and Meta-analysis". Annals of Emergency Medicine. 69 (3): 353–361.e3. doi:10.1016/j.annemergmed.2016.06.044. PMID 27616037. Archived from the original on 18 June 2020. Retrieved 10 July 2019.
- ↑ 5.0 5.1 5.2 5.3 5.4 5.5 "7. Genito-urinary system". British National Formulary (BNF) (82 ed.). London: BMJ Group and the Pharmaceutical Press. September 2021 – March 2022. pp. 831–833. ISBN 978-0-85711-413-6.
- ↑ 6.0 6.1 "WHOCC - ATC/DDD Index". www.whocc.no. Archived from the original on 22 January 2021. Retrieved 9 September 2020.
- ↑ Hutchison, Lisa C.; Sleeper, Rebecca B. (2010). Fundamentals of Geriatric Pharmacotherapy: An Evidence-Based Approach. ASHP. p. 209. ISBN 9781585283057. Archived from the original on 24 December 2018. Retrieved 24 December 2018.
- ↑ "NADAC as of 2018-12-19". Centers for Medicare and Medicaid Services. Archived from the original on 19 December 2018. Retrieved 22 December 2018.
- ↑ Hitchings, Andrew; Lonsdale, Dagan; Burrage, Daniel; Baker, Emma (2019). The Top 100 Drugs: Clinical Pharmacology and Practical Prescribing (2nd ed.). Elsevier. pp. 26–27. ISBN 978-0-7020-7442-4. Archived from the original on 22 May 2021. Retrieved 9 November 2021.
- ↑ 10.0 10.1 "The Top 300 of 2020". ClinCalc. Archived from the original on 18 March 2020. Retrieved 11 April 2020.
- ↑ 11.0 11.1 "Tamsulosin Hydrochloride - Drug Usage Statistics". ClinCalc. 23 December 2019. Archived from the original on 12 April 2020. Retrieved 11 April 2020.
- ↑ "Tamsulosin Aids Stone Expulsion". Renal and Urology News. 7 January 2011. Archived from the original on 14 May 2012. Retrieved 4 October 2012.
- ↑ "Study Shows Use of Tamsulosin or Nifedipine Helps Patients to Clear Ureteral Stone Fragments Faster and Reduces Rate of Recurrence". Archived from the original on 5 March 2014. Retrieved 4 October 2012.
- ↑ Lucas MG, Stephenson TP, Nargund V (February 2005). "Tamsulosin in the management of patients in acute urinary retention from benign prostatic hyperplasia". BJU Int. 95 (3): 354–7. doi:10.1111/j.1464-410X.2005.05299.x. PMID 15679793.
- ↑ 15.0 15.1 15.2 Lewis, Sharon Mantik. Medical-surgical nursing : assessment and management of clinical problems. Dirksen, Shannon Ruff,, Heitkemper, Margaret M. (Margaret McLean),, Bucher, Linda,, Harding, Mariann (Ninth ed.). St. Louis, Missouri. ISBN 978-0-323-10089-2. OCLC 228373703.
- ↑ Roehrborn CG, Siami P, Barkin J, et al. (February 2008). "The effects of dutasteride, tamsulosin and combination therapy on lower urinary tract symptoms in men with benign prostatic hyperplasia and prostatic enlargement: 2-year results from the CombAT study". J. Urol. 179 (2): 616–21, discussion 621. doi:10.1016/j.juro.2007.09.084. PMID 18082216.
- ↑ FDA approval letter Archived 26 July 2015 at the Wayback Machine at FDA.gov
- ↑ Australian Medicines Handbook[full citation needed]
- ↑ "Tamsulosin (Oral Route) - Before Using". Mayo Clinic. Archived from the original on 3 May 2015. Retrieved 5 June 2015.
- ↑ Medscape, Good Cataract Surgery Outcomes Possible in Intraoperative Floppy Iris Syndrome Due to Tamsulosin Archived 10 December 2012 at the Wayback Machine.
- ↑ 21.0 21.1 Bird, ST; Delaney, JA; Brophy, JM; Etminan, M; Skeldon, SC; Hartzema, AG (5 November 2013). "Tamsulosin treatment for benign prostatic hyperplasia and risk of severe hypotension in men aged 40-85 years in the United States: risk window analyses using between and within patient methodology". BMJ (Clinical Research Ed.). 347: f6320. doi:10.1136/bmj.f6320. PMC 3817852. PMID 24192967.
- ↑ Ramirez, J (5 November 2013). "Severe hypotension associated with α blocker tamsulosin". BMJ (Clinical Research Ed.). 347: f6492. doi:10.1136/bmj.f6492. hdl:10906/78488. PMID 24192968.
- ↑ Page RL, 2nd; O'Bryant, CL; Cheng, D; Dow, TJ; Ky, B; Stein, CM; Spencer, AP; Trupp, RJ; Lindenfeld, J; American Heart Association Clinical Pharmacology and Heart Failure and Transplantation Committees of the Council on Clinical Cardiology; Council on Cardiovascular Surgery and Anesthesia; Council on Cardiovascular and Stroke Nursing; and Council on Quality of Care and Outcomes, Research. (9 August 2016). "Drugs That May Cause or Exacerbate Heart Failure: A Scientific Statement From the American Heart Association". Circulation. 134 (6): e32–69. doi:10.1161/CIR.0000000000000426. PMID 27400984.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ↑ "Tamsulosin Side Effects". Drugs.com. Archived from the original on 1 March 2011. Retrieved 27 April 2011.
- ↑ Shen, Howard (2008). Illustrated Pharmacology Memory Cards: PharMnemonics. Minireview. p. 13. ISBN 978-1-59541-101-3.
- ↑ "Flomax – Big Patent Expirations of 2010". 10 February 2010. Archived from the original on 22 April 2012. Retrieved 14 January 2012.
- ↑ "FDA Approves First Generic Tamsulosin to Treat Enlarged Prostate Gland" (Press release). U.S. Food and Drug Administration (FDA). 2 March 2010. Archived from the original on 11 August 2016. Retrieved 16 December 2019.
- ↑ "OTC tamsulosin for benign prostatic hyperplasia". Drug and Therapeutics Bulletin. 48 (10): 113–6. October 2010. doi:10.1136/dtb.2010.10.0052. PMID 20926447.
- ↑ Dr. Sandro Magnanelli; Dr.ssa Ada Maria Vetere. "Pradif 0,4 Mg Capsule Rigide A Rilascio Prolungato". Torrinomedica.it. Archived from the original on 21 June 2013. Retrieved 15 November 2012.
- ↑ Mylan. "Tamsulosina Mylan 0,4 mg cápsulas duras de liberación modificada EFG" (PDF). cima.aemps.es. Archived (PDF) from the original on 29 October 2018. Retrieved 29 October 2018.
- ↑ "Drugs.com Database". Archived from the original on 5 August 2017. Retrieved 23 January 2018.
- ↑ "Novartis hits Astellas with transplant drug generic". Reuters. 11 August 2009. Archived from the original on 29 August 2021. Retrieved 11 August 2009.
External links
| Identifiers: |
|
|---|
- "Tamsulosin". Drug Information Portal. U.S. National Library of Medicine. Archived from the original on 12 April 2020. Retrieved 12 April 2020.
- Pages using duplicate arguments in template calls
- Webarchive template wayback links
- All articles with incomplete citations
- Articles with incomplete citations from September 2016
- Articles with invalid date parameter in template
- CS1 maint: multiple names: authors list
- Use dmy dates from April 2017
- Chem-molar-mass both hardcoded and calculated
- Chemical articles with unknown parameter in Infobox drug
- Chemical articles without CAS registry number
- Articles without EBI source
- Chemical pages without ChemSpiderID
- Chemical pages without DrugBank identifier
- Articles without KEGG source
- Articles without UNII source
- Drugs missing an ATC code
- Drugboxes which contain changes to watched fields
- Articles with hatnote templates targeting a nonexistent page
- All articles with unsourced statements
- Articles with unsourced statements from August 2017
- Alpha blockers
- Phenethylamines
- Phenol ethers
- Sulfonamides
- Astellas Pharma
- RTT