User:Project Osprey/sandbox

In organic chemistry, organophosphates (also known as phosphate esters, or OPEs) are a class of organophosphorus compounds with the general structure O=P(OR)3, a central phosphate molecule with alkyl or aromatic substituents.[1] They can be considered as esters of phosphoric acid.
Like most functional groups, organophosphates occur in a diverse range of forms,[2] with important examples including key biomolecules such as DNA, RNA and ATP, as well as many insecticides, herbicides, nerve agents and flame retardants. OPEs have been widely used in various products as flame retardants, plasticizers, and performance additives to engine oil. The popularity of OPEs as flame retardants came as a substitution for the highly regulated brominated flame retardants.[3] The low cost of production and compatibility to diverse polymers made OPEs to be widely used in industry including textile, furniture, electronics as plasticizers and flame retardants. These compounds are added to the final product physically rather than by chemical bond.[4] Due to this, OPEs leak into the environment more readily through volatilization, leaching, and abrasion.[5] OPEs have been detected in diverse environmental compartments such as air, dust, water, sediment, soil and biota samples at higher frequency and concentration.[1][5]
bits
EP138464
https://phosphorusplatform.eu/images/download/ESPP-NNP-DPP_nutrient-recovery_tech_catalogue.pdf
Top level [6]
Nomenclature https://iupac.qmul.ac.uk/misc/phospho.html
Organophosphate Esters in the Environment from Biological Effects to Distribution[10]
==new
Early paper on hydrolysis[11]
Review, emerging concern[12]
Tox review, but low-ranking journal, trace the refs[13]
Reactions
unsorted refs
Mechanism 2011[15]
DNA?[16] phosphodiester bond DNA no enzyme hydrolysis[17] Rare secondary metabolites?[18]
Prodrugs of biologically active phosphate esters[19]
Understanding phosphoryl and sulfuryl transfer is central to many biochemical processes. However, despite decades of experimental and computational studies, a consensus concerning the precise mechanistic details of these reactions has yet to be reached.[20]
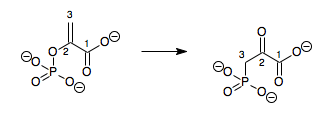
Metaphosphate is an intermediate in the hydrolysis of phosphate esters but it is difficult to isolate, as it readily hydrolyses to from a dihydrogen phosphate ion ([H
2PO
4]−
) and tends to self-react in the absence of water to form rings or infinite chains:[21]
Text begins
Few reactions exist which can disrupt the PO4 core of organophosphates. As such The chemistry of phosphate esters is effectively limited to either hydrolysis or transesterification (phosphoryl transfer). With some exceptions, the rates of these reactions are exceedingly slow in the absence of catalysts.
Determining the precise mechanisms by which organophosphates undergo hydrolysis or phosphoryl transfer has been the subject of huge amounts of research since the 1950s. The transfer of phosphoryl groups from one entity to another plays a central role in biosynthesis, with approximately one-third of all proteins in the cell being phosphorylated at any given time.[22] The phosphates diesters which form the backbone of DNA and RNA are also chiral and understanding how they are assembled enantioselectively is necessary part of understand the synthesis of these biomolecules.
hydrolysis
- A concerted SN2-type process where the transition state is not an isolatible intermediate. This reaction proceeds with complete inversion of configuration at the phosphorus atom.
- An associative substitution process, where a nucleophile attacks to from an intermediate 5-coordinate phosphorane with a trigonal bipyramidal geometry. This reaction generally proceeds with inversion of configuration, but the longer the intermediate lasts the more pseudorotation will scramble the configuration.
- A dissociative substitution with initial loss of hydroxide leaving 3-coordinate metaphosphate ion. one face of the phosphate can still be blocked by the leaving group
Classically the leaving group departs from the opposite face as the attacking nucleophile. Pseudorotation
decrease in total bond order between phosphorus and the nucleophile and leaving group are called loose transition states.
complete inversion of configuration
subject of intensive study since early as the 1950s,[23]
mono‑ di‑ and tri‑esters all undergo hydrolysis via a concerted mechanism
Enzymatic phosphoryl-transfer reactions
the first-order rate constant for attack on methyl phosphate dianion by water is less than 10–20 s–1[27] a half-life of more than one trillion years. Alkaline phosphatase catalyzes this hydrolysis with a second-order rate constant (kcat/KM) of 1.2 × 106 M–1s–1[28], giving a rate acceleration of more than 1027-fold
enzymes catalyzing phopshoryl transfer that can function at both high and low pH, enzymes that utilize direct attack by water or alternately employ an enzyme-derived nucleophile, as well as enzymes that employ metal ions in catalysis, and others that do not.
With regard to organophosphate poisoning

The slowness of hydrolysis accounts in part for the toxicity of organophosphates as it causes them to act as irreversible acetylcholinesterase inhibitors
N-OH groups like hydroxylamines and oximes react much faster and form the basis of treatments for organophosphate poisoning such as pralidoxime asoxime chloride

Other processes
phospha-Fries rearrangement[30]
Biosynthesis of phosphonates and phosphinates[31] examples include phosphoenolpyruvate mutase
Forms
Organophosphates are a class of compounds encompasing a number of distinct but closely related function groups, primarily the monoesters, diesters and triesters of phosphoric acid. In general man-made organophosphates are most often triesters, while biological organophosphates are usually mono- or di-esters. The hydolysis of triesters can form diesters and monoesters.[32]
In the context of pesticides, derivatives of organophosphates such as [[[organothiophosphate]]s (P=S) or phosphorodiamidates (P-N) are classed as being organophosphates. The reason is that these compound are converted into organophosphates biologically and thus share a similar mode-of-action, which is cholinesterase inhibition.
In biology the esters of diphosphoric acid and triphosphoric acid are generally included as organophosphates. The reason is again a practical one, as many cellular processes involve the mono- di and tri- phosphates of the same compound. For instance, the phosphates of adenosine (AMP, ADP, ATP) play a key role in many metabolic processes.
Synthesis
- Alcoholysis of POCl3
Phosphorus oxychloride reacts readily with alcohols to give organophosphates. This is the dominate industrial route and is responsible for almost all organophosphate production.
- O=PCl3 + 3 ROH → O=P(OR)3 + 3 HCl
When aliphatic alcohols are used the HCl by-product can react with the phosphate esters to give organochlorides and a lower ester. This reaction is usually undesirable and is exacerbated by high reaction temperatures, but it can be inhibited by the prompt removal of HCl or the use of a base.
- O=P(OR)3 + HCl → O=P(OR)2OH + RCl
- Esterification of phosphoric acid and P2O5
Esterifications of phosphoric acid with alcohols proceed less readily than the more common carboxylic acid esterifications, with the reactions rarely proceeding much further than the phosphate mono-ester. The reaction requires high temperatures, under which the phosphoric acid can dehydrate to form poly-phosphoric acids. These are exceedingly viscous and their linear polymeric structure renders them less reactive than phosphoric acid.[33] Despite these limitations the reaction does see industrial use for the formation of monoalkyl phosphates, which are used as surfactants.[34] A major appeal of this this route is the low cost of phosphoric acid compared to phosphorus oxychloride.
- OP(OH)3 + ROH → OP(OH)2(OR) + H2O
P2O5 is the anhydride of phosphoric acid and acts similarly. The reaction yields equimolar amounts of di- and monoesters with no phosphoric acid. The process is mostly limited to primary alcohols, as secondary alcohols are prone to undesirable side reactions such as dehydration.[35]
- Oxidation of phosphite esters
Organophosphites can be easily oxidised to give organophosphates. This might ordinarily be considered a specialised method, however large quantities of organophosphites are produced as antioxidant stabilisers for plastics, with their gradual oxidation forming organophosphates in the human environment.[36]
- P(OR)3 + [O] → OP(OR)3
- Phosphorylation
The formation of organophosphates is an important part of biochemistry and living systems achieve this using a variety of enzymes. Phosphorylation is essential to the processes of both anaerobic and aerobic respiration, which involve the production of adenosine triphosphate (ATP), the "high-energy" exchange medium in the cell.
Properties
Bonding
The bonding in organophosphates has been a matter of prolonged debate; the phosphorous atom is classically hypervalent, as it possesses more bonds than the octet rule should allow.[38] The focus of debate is usually on the nature of the phosphoryl P=O bond, which displays (in spite of the common depiction) non-classical bonding, with a bond order somewhere between 1 and 2. Early papers explained the hypervalence in terms of d-orbital hybridisation, with the energy penalty of promoting electrons into the higher energy orbitals being off-set by the stabilisation of additional bonding.[39] Later advances in computational chemistry showed that d-orbitals played little significant role in bonding.[40][41] Current models rely on either negative hyperconjugation,[42] or a more complex arraignment with a dative-type bond from P to O, combined with back-donation from a 2p orbital on the oxygen.[41][43] These models agree with the experimental observations of the phosphoryl as being shorter than P-OR bonds[44] and much more polarised. It has been argued that a more accurate depiction is dipolar (i.e. (RO)3P+-O-),[45] which is similar to the depiction of phosphorous ylides such as methylenetriphenylphosphorane. However in contrast to ylides, the phosphoryl group is unreactive and organophosphates are poor nucleophiles, despite the high concentration of charge on the phosphoryl oxygen. The polarisation accounts in part for the higher melting points of phosphates when compared to their corresponding phosphites. The bonding in penta-coordinate phosphoranes (i.e. P(OR)5) is entirely different and involves three-center four-electron bonds.
Acidity
Phosphate esters bearing OH groups are acidic. The pKa of the first OH group is typically between 1-2, with the second OH deprotonating at a pKa of 6-7.[46] This is great practical importance as it means that phosphate mono- and di-esters are negatively charged at physiological pH. This includes biomolecules such as DNA and RNA. The presence of this negative charge make these compound much more water soluble and more resistant to degradation by hydrolysis or other forms of nucleophilic attack.
Water solubility
The water solubility of organophosphates is important factor is important in biological, industrial and environmental settings.
The wide variety of substitutes used in organophosphate esters results in great variations in physical properties. OPEs exhibit a wide range of octanol water partitioning coefficient where log Kow values range from -0.98 up to 10.6.[3] The predominant OPEs used as flame retardants and plasticizers have a positive log Kow values ranging between 1.44 and 9.49 signifying hydrophobicity.[3][47][5][10] Thus, owing to this hydrophobicity OPEs are presumptively bioaccumulated and biomagnified in aquatic ecosystems.[4] Halogenated organophosphates tend to be denser than water and sink [48]
Persistence
Laboratory experiments had shown that the non-halogenated OPEs are prone to photolysis, while the chlorinated OPEs such as TCEP and TCPP, however, seemed to be resistant to degradation by sunlight.[3]
Industrial materials
Pesticides

Organophosphates are best known for their use as pesticides. The vast majority are insecticides and are used either to protect crops, or as vector control agents to reduce the transmission of diseases spread by insects, such as mosquitoes. Health concerns have seen their use significantly decrease since the turn of the century.[49][50] Glyphosate is sometimes called an organophosphate, but is in-fact a phosphonate. It's chemistry, mechanism of toxicity and end-use as a herbicide are different from the organophosphate insecticides.
The development of organophosphate insecticides dates back to the 1930s and is generally credited to Gerhard Schrader.[51] At the time pesticides were largely limited to arsenic salts (calcium arsenate, lead arsenate and Paris green)[52] or pyrethrin plant extracts, all of which had major problems.[53] Schrader was seeking more effective agents, however while some organophosphates were found to be far more dangerous to insects than higher animals,[54] the potential effectiveness of others as chemical weapons did not go unnoticed. The development of organophosphate insecticides and the earliest nerve agents was conjoined, with Schrader also developing the nerve agents tabun and sarin. Organophosphate pesticides were not commercialised until after WWII. Parathion was among the first marketed, followed by malathion and azinphosmethyl . Although organophosphates were used in considerable qualities they were originally less important than organochlorine insecticides such as DDT, dieldrin, and heptachlor. When many of the organochlorines were banned in the 1970s, following the publishing of Silent Spring, organophosphates became the most important class of insecticides globally. Nearly 100 were commercialised, with the following being a varied selection:
- Acephate
- Azinphos-methyl
- Bensulide
- Chlorethoxyfos
- Coumaphos
- Diazinon
- Dichlorvos
- Dicrotophos
- Dimethoate
- Disulfoton
- Ethion
- Ethoprop
- Ethyl parathion
- Fenamiphos
- Fenitrothion
- Fonofos
- Isoxathion
- Malathion
- Methamidophos
- Methidathion
- Mevinphos
- Naled
- Phosmet
- Profenofos
- Propetamphos
- Sulfotep
- Tebupirimfos
- Temephos
- Terbufos
- Tetrachlorvinphos
- Triazofos
- Trichlorfon
Organophosphate insecticides are acetylcholinesterase inhibitors, and when introduced to an organism they act to fatally disrupt the transmission of nerve signals. The risk of human death through organophosphate poisoning[55] was obvious from the start and let to efforts to lower toxicity again mammals while not reducing efficacy again insects.[56][57]
The majority of organophosphate insecticides are organothiophosphates (P=S) or phosphorodiamidates (P-N), both of which are significantly weaker acetylcholinesterase inhibitors than the corresponding phosphates (P=O). They are 'activated' biologically by the exposed organism, via oxidative conversion of P=S to P=O,[58] hydroxylation,[59][60], or other related process which see them transformed into organophosphates. In mammals these transformations occur almost exclusively in the liver,[61] while in insects they take place in the gut and fat body.[62][63][64] As the transformations are handled by different enzymes in different classes of organism it is possible to find compounds which activate more rapidly and completely in insects, and thus display more targeted lethal action.
This selectivity is far from perfect and organophosphate insecticides remain acutely toxic to humans, with many thousands estimated to be killed each year due to intentional[65] or unintentional poisoning. Beyond their acute toxicity, exposure to organophosphates is associated with a number of heath risks, including organophosphate-induced delayed neuropathy (muscle weakness) and developmental neurotoxicity.[51][66][67] There is limited evidence that certain compounds cause cancer, including malathion and diazinon.[68] Children[69] and farmworkers[70] are considered to be at greater risk.
Pesticide regulation in the United States and the regulation of pesticides in the European Union have both been increasing restrictions on organophosphate pesticides since the 1990s, particularly when used for crop protection. The use of organophosphates has decreased considerably since that time, having been replaced by pyrethroids and neonicotinoids, which are effective a much lower levels.[71] Reported cases of organophosphate poisoning in the US have reduced during this period.[72][73] Regulation in the global south can be less extensive.[74][75]
In 2015, only 3 of the 50 most common crop-specific pesticides used in the US were organophosphates (Chlorpyrifos, Bensulide, Acephate),[76] of these Chlorpyrifos was banned in 2021.[77] No new organophosphate pesticides have been commercialised in the 21st century.[78] The situation in vector control is fairly similar, despite different risk trade-offs,[79] with the global use of organophosphate insecticides falling by nearly half between 2010 and 2019.[50] Pirimiphos-methyl, Malathion and Temefos are still important, primarily for the control of malaria in the Asia-Pacific region.[50] The continued use of these agents is being challenged by the emergence of insecticide resistance.[80]
Flame retardants
Flame retardants are added to materials to prevent combustion and to delay the spread of fire after ignition. Organophosphate flame retardants are part of a wider family of phosphorous-based agents which include organic phosphonate and phosphinate esters, in addition to inorganic salts.[81][82] When some prominent brominated flame retardant were banned in the early 2000s phosphorous-based agents were promoted as safer replacements. This has led to a large increase in their use, with an estimated 1 million tonnes of organophosphate flame retardants produced in 2018.[83] Safety concerns have subsequently been raised about some of these reagents,[84][85] with several under regulatory scrutiny.[86][87]
Organophosphate flame retardants were first developed in the first half of the twentieth century in the from of triphenyl phosphate, tricresyl phosphate and tributyl phosphate for use in plastics like cellulose nitrate and cellulose acetate.[88] Use in cellulose products is still significant, but the largest area of application is now in plasticized vinyl polymers, principally PVC. The more modern organophosphate flame retardants come in 2 major types; chlorinated aliphatic compounds or aromatic diphosphates.[81] The chlorinated compounds TDCPP, TCPP and TCEP are all involatile liquids, of which TCPP is perhaps the most important. They are used in polyurethane (soft furnishings), PVC (wire and cable) phenolic resins and epoxy resinss (varnishes, coatings and adhesives). The most important of the diphosphates is bisphenol-A bis(diphenyl phosphate), with related analogues based around resorcinol and hydroquinone. These are used in polymer blends of engineering plastics, such as PPO/HIPS and PC/ABS,[89] which are commonly used to make casing for electrical items like TVs, computers and home appliances.
Organophosphates act multifunctionally to retard fire in both the gas phase and condensed (solid) phase. Halogenated organophosphates are more active overall as their degradation products interfere with combustion directly in the gas phase. All organophosphates have activity in the condensed phase, by forming phosphorus acids which promote char formation, insulating the surface from heat and air.
Organophosphates were originally thought to be a safe replacements for brominated flame retardants, however many are now coming under regulatory pressure due to their apparent health risks.[87][90][91] The chlorinated organophosphates may be carcinogenic, while others such as tricresyl phosphate have necrotoxic properties.[92] Bisphenol-A bis(diphenyl phosphate) can hydrolyse to form Bisphenol-A which is under significant scrutiny as potential endocrine-disrupting chemical. Although their names imply that they are single discreet species the some compounds are produced as complex mixtures, for instance commercial grade TCPP can contain 7 different isomers,[93] while tricresyl phosphate can contain up to 10.[94] This makes their safety profiles harder to ascertain, as material from different producers can have different compositions.[95]
Plasticisers
Plasticisers are added to polymers and plastics to improve their flexibility and processability, giving a softer more easily deformable material. In this way brittle polymers can be made more durable. Organophosphates find use because they are multifunctional; primarily plasticising but also imparting flame resistance. The most frequently plasticised polymers are the vinyls (PVC, PVB, PVA and PVCA), as well as cellulose plastics (cellulose acetate, nitrocellulose and cellulose acetate butyrate).[96] PVC dominates the market, consuming 80-90% of global plasticiser production.[96][97] PVC can accept large amounts of plasticiser; a PVC item may be 70-80% plasticiser by mass in extreme cases, but loadings of between 0-50% are more common.[98] The main applications of these products are in wire and cable insulation, flexible pipe, automotive interiors, plastic sheeting, vinyl flooring, and toys.
Pure PVC is more than 60% chlorine by mass and difficult to burn, but its flammability increases the more it is plasticised.[99] Organophosphates can act as both plasticisers and flame retarders. Compounds used are typically triaryl or alkyl diaryl phosphates, with cresyl diphenyl phosphate and 2-ethylhexyl diphenyl phosphate being important respective examples type.[100] These are both liquids with high boiling points. Organophosphates are more expensive than traditional plasticisers and so tend used in combination with other plasticisers and flame retardants.[101]
Hydraulic fluids and lubricant additives
Similar to their use as plastisiers, organophosphates are well suited to use as hydraulic fluids due to their low freezing points and high boiling points, fire-resistance, non-corrosiveness, excellent boundary lubrication properties and good general chemical stability. The triaryl phosphates are the most important group, with tricresyl phosphate being the first to be commercialised in the 1940s, with trixylyl phosphate following shortly after. Butylphenyl diphenyl phosphate and propylphenyl diphenyl phosphate became available after 1960.[102]
In addition to their use as hydraulic base-stock, organophosphates (tricresyl phosphate) and metal organothiophosphates (zinc dithiophosphate) are used as both an antiwear additives and extreme pressure additives in lubricants, where they remain effective even at high temperatures.[103][104][105]
Metal extractants
Organophosphates have long been used in the field of extractive metallurgy to liberate valuable rare earths from their ores.[106] Di(2-ethylhexyl)phosphoric acid and tributyl phosphate are used for the liquid–liquid extraction of these elements from the acidic mixtures form by the leaching of mineral deposits.[107] These compounds are also used for the PUREX (plutonium uranium reduction extraction) process, which is used for nuclear reprocessing.[108]
Surfactants
Mono- and di- phosphate esters of alcohols or alcohol ethoxylates are used as surfactants.[109] Compared to the more common sulfur-based anionic surfactants (such as LAS or SLES), phosphate ester surfactants are more expensive and generate less foam.[109] Benefits include stability over a broad pH range, low skin irritation and a high tolerance to dissolved salts.[110] In agricultural settings monoesters of fatty alcohol ethoxylates are used, which are able to disperse poorly miscible or insoluble pesticides into water. As they are low-foaming these mixtures can be sprayed effectively onto fields, while a high salt tolerance allows co-spraying of pesticides and inorganic fertilisers.[111] Low-levels of phosphate mono-esters, such as potassium cetyl phosphate, find use in cosmetic creams and lotions.[112] These in oil-in-water formulations are primarily based on non-ionic surfactants, with the anionic phosphate acting as emulsion-stabilisers. Phosphate tri-esters such as tributyl phosphate are used as anti-foaming agent in paints and concrete.
Nerve agents
Although the first phosphorous compounds observed to act as cholinesterase inhibitors were organophosphates,[113] the vast majority of nerve agents are instead phosphonates containing a P-C bond. Only a handful of organophosphate nerve agents were developed between the 1930s and 1960s, including diisopropylfluorophosphate, VG and NPF. Between 1971 and 1993 the Soviet Union developed many new potential nerve agents, commonly known as the Novichok agents.[114] Some of these can be considered organophosphates (in a broad sense), being derivatives of fluorophosphoric acid. Examples include A-232, A-234, A-262, C01-A035 and C01-A039. The most notable of these in A-234, which was claimed to be responsible for the poisoning of Sergei and Yulia Skripal in Salisbury (UK) 2018.[115]
Bio-molecules
Monoesters Adenosine monophosphate Fludarabine phosphate Cytidine 5′-monophosphate Phytic acid Pyridoxal 5′-phosphate 5′-UMP Guanosine monophosphate NADP Glucose 6-phosphate Inosinic acid Sphingosine-1-phosphate
10-Methacryloyloxydecyl dihydrogen phosphate dental
Diesters Cytidylylguanosine Miltefosine Dodecylphosphocholine Perifosine CMP-Neu5Ac

Guanitoxin is a naturally occurring organophosphate produced by cyanobacteria.
The detection of OPEs in the air as far away as Antarctica at concentrations around 1 ng/m3 suggests their persistence in air, and their potential for long-range transport.[10] OPEs were measured in high frequency in air and water and widely distributed in northern hemisphere.[118][119] The chlorinated OPEs (TCEP, TCIPP, TDCIPP) in urban sampling sites and non-halogenated like TBOEP in rural areas respectively were frequently measured in the environment across multiple sites. In the Laurentian Great Lakes total OPEs concentrations were found to be 2–3 orders of magnitude higher than concentrations of brominated flame retardants measured in similar air.[119] Waters from rivers in Germany, Austria, and Spain have been consistently recorded for TBOEP and TCIPP at highest concentrations.[10] From these studies, it is clear that OPE concentrations in both air and water samples are often orders of magnitude higher than other flame retardants, and that concentrations are largely dependent on sampling location, with higher concentrations in more urban, polluted locations.
References
- ^ a b Greaves, Alana K.; Letcher, Robert J.; Chen, Da; McGoldrick, Daryl J.; Gauthier, Lewis T.; Backus, Sean M. (2016-10-01). "Retrospective analysis of organophosphate flame retardants in herring gull eggs and relation to the aquatic food web in the Laurentian Great Lakes of North America". Environmental Research. 150: 255–263. Bibcode:2016ER....150..255G. doi:10.1016/j.envres.2016.06.006. ISSN 0013-9351. PMID 27322497.
- ^ Ung, Sosthène P.-M.; Li, Chao-Jun (2023). "From rocks to bioactive compounds: a journey through the global P( v ) organophosphorus industry and its sustainability". RSC Sustainability. 1 (1): 11–37. doi:10.1039/D2SU00015F.
- ^ a b c d Veen, Ike van der; Boer, Jacob de (2012). "Phosphorus flame retardants: Properties, production, environmental occurrence, toxicity and analysis". Chemosphere. 88 (10): 1119–1153. Bibcode:2012Chmsp..88.1119V. doi:10.1016/j.chemosphere.2012.03.067. PMID 22537891.
- ^ a b Wang, Xiaolei; Zhong, Wenjue; Xiao, Bowen; Liu, Qing; Yang, Liping; Covaci, Adrian; Zhu, Lingyan (2019-04-01). "Bioavailability and biomagnification of organophosphate esters in the food web of Taihu Lake, China: Impacts of chemical properties and metabolism". Environment International. 125: 25–32. doi:10.1016/j.envint.2019.01.018. ISSN 0160-4120. PMID 30690428.
- ^ a b c Wei, Gao-Ling; Li, Ding-Qiang; Zhuo, Mu-Ning; Liao, Yi-Shan; Xie, Zhen-Yue; Guo, Tai-Long; Li, Jun-Jie; Zhang, Si-Yi; Liang, Zhi-Quan (January 2015). "Organophosphorus flame retardants and plasticizers: Sources, occurrence, toxicity and human exposure". Environmental Pollution. 196: 29–46. doi:10.1016/j.envpol.2014.09.012. PMID 25290907.
- ^ Weferling, Norbert; Zhang, Shuan Ming; Chiang, Cho Hsien (2016). "Commercial Organophosphorus Chemicals: Status and New Developments". Procedia Engineering. 138: 291–301. doi:10.1016/j.proeng.2016.02.087.
- ^ Ung, Sosthène P.-M.; Li, Chao-Jun (2023). "From rocks to bioactive compounds: a journey through the global P( v ) organophosphorus industry and its sustainability". RSC Sustainability. 1 (1): 11–37. doi:10.1039/d2su00015f.
- ^ Mew, Michael; Steiner, Gerald; Geissler, Bernhard (5 April 2018). "Phosphorus Supply Chain—Scientific, Technical, and Economic Foundations: A Transdisciplinary Orientation". Sustainability. 10 (4): 1087. doi:10.3390/su10041087.
- ^ Lao, Jia-Yong; Ruan, Yuefei; Leung, Kenneth M. Y.; Zeng, Eddy Y.; Lam, Paul K. S. (3 April 2023). "Review on age-specific exposure to organophosphate esters: Multiple exposure pathways and microenvironments". Critical Reviews in Environmental Science and Technology. 53 (7): 803–826. doi:10.1080/10643389.2022.2087428.
- ^ a b c d Greaves, Alana K.; Letcher, Robert J. (January 2017). "A Review of Organophosphate Esters in the Environment from Biological Effects to Distribution and Fate". Bulletin of Environmental Contamination and Toxicology. 98 (1): 2–7. doi:10.1007/s00128-016-1898-0. ISSN 0007-4861. PMID 27510993. S2CID 19824807.
- ^ Westheimer, F. H.; Huang, Shaw.; Covitz, Frank. (January 1988). "Rates and mechanisms of hydrolysis of esters of phosphorous acid". Journal of the American Chemical Society. 110 (1): 181–185. doi:10.1021/ja00209a029.
- ^ Ye, Langjie; Li, Jianhua; Gong, Shuai; Herczegh, Sofia M.; Zhang, Qi; Letcher, Robert J.; Su, Guanyong (October 2023). "Established and emerging organophosphate esters (OPEs) and the expansion of an environmental contamination issue: A review and future directions". Journal of Hazardous Materials. 459: 132095. doi:10.1016/j.jhazmat.2023.132095.
- ^ Aroniadou-Anderjaska, Vassiliki; Figueiredo, Taiza H.; de Araujo Furtado, Marcio; Pidoplichko, Volodymyr I.; Braga, Maria F. M. (18 October 2023). "Mechanisms of Organophosphate Toxicity and the Role of Acetylcholinesterase Inhibition". Toxics. 11 (10): 866. doi:10.3390/toxics11100866.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Kirby, Anthony J.; Nome, Faruk (21 July 2015). "Fundamentals of Phosphate Transfer". Accounts of Chemical Research. 48 (7): 1806–1814. doi:10.1021/acs.accounts.5b00072.
- ^ Lassila, Jonathan K.; Zalatan, Jesse G.; Herschlag, Daniel (7 July 2011). "Biological Phosphoryl-Transfer Reactions: Understanding Mechanism and Catalysis". Annual Review of Biochemistry. 80 (1): 669–702. doi:10.1146/annurev-biochem-060409-092741.
- ^ Müller, Sabine (June 2017). "Phosphorous chemistry in vivo: what makes the phosphoesters in DNA and RNA so diverse?". ChemTexts. 3 (2). doi:10.1007/s40828-017-0046-8.
- ^ Schroeder, Gottfried K.; Lad, Chetan; Wyman, Paul; Williams, Nicholas H.; Wolfenden, Richard (14 March 2006). "The time required for water attack at the phosphorus atom of simple phosphodiesters and of DNA". Proceedings of the National Academy of Sciences. 103 (11): 4052–4055. doi:10.1073/pnas.0510879103.
- ^ Della-Felice, Franco; de Andrade Bartolomeu, Aloisio; Pilli, Ronaldo Aloise (2022). "The phosphate ester group in secondary metabolites". Natural Product Reports. 39 (5): 1066–1107. doi:10.1039/D1NP00078K.
- ^ Schultz, Carsten (March 2003). "Prodrugs of biologically active phosphate esters". Bioorganic & Medicinal Chemistry. 11 (6): 885–898. doi:10.1016/S0968-0896(02)00552-7.
- ^ Duarte, Fernanda; Åqvist, Johan; Williams, Nicholas H.; Kamerlin, Shina C. L. (28 January 2015). "Resolving Apparent Conflicts between Theoretical and Experimental Models of Phosphate Monoester Hydrolysis". Journal of the American Chemical Society. 137 (3): 1081–1093. doi:10.1021/ja5082712.
- ^ Regitz, Manfred; Maas, Gerhard (1981). "Short-lived phosphorus(V) compounds having coordination number 3". Organic Chemistry. 97: 71–120. doi:10.1007/BFb0037041.
- ^ Manning, G.; Whyte, D. B.; Martinez, R.; Hunter, T.; Sudarsanam, S. (6 December 2002). "The Protein Kinase Complement of the Human Genome". Science. 298 (5600): 1912–1934. doi:10.1126/science.1075762.
- ^ Bunton, C. A.; Llewellyn, D. R.; Oldham, K. G.; Vernon, C. A. (1958). "716. The reactions of organic phosphates. Part I. The hydrolysis of methyl dihydrogen phosphate". Journal of the Chemical Society (Resumed): 3574. doi:10.1039/JR9580003574.
- ^ Lassila, Jonathan K.; Zalatan, Jesse G.; Herschlag, Daniel (7 July 2011). "Biological Phosphoryl-Transfer Reactions: Understanding Mechanism and Catalysis". Annual Review of Biochemistry. 80 (1): 669–702. doi:10.1146/annurev-biochem-060409-092741. PMC 3418923.
- ^ Kamerlin, Shina C. L.; Sharma, Pankaz K.; Prasad, Ram B.; Warshel, Arieh (February 2013). "Why nature really chose phosphate". Quarterly Reviews of Biophysics. 46 (1): 1–132. doi:10.1017/S0033583512000157.
- ^ Kirby, Anthony J.; Nome, Faruk (21 July 2015). "Fundamentals of Phosphate Transfer". Accounts of Chemical Research. 48 (7): 1806–1814. doi:10.1021/acs.accounts.5b00072.
- ^ Lad, Chetan; Williams, Nicholas H.; Wolfenden, Richard (13 May 2003). "The rate of hydrolysis of phosphomonoester dianions and the exceptional catalytic proficiencies of protein and inositol phosphatases". Proceedings of the National Academy of Sciences. 100 (10): 5607–5610. doi:10.1073/pnas.0631607100.
- ^ Zalatan, Jesse G.; Fenn, Timothy D.; Herschlag, Daniel (December 2008). "Comparative Enzymology in the Alkaline Phosphatase Superfamily to Determine the Catalytic Role of an Active-Site Metal Ion". Journal of Molecular Biology. 384 (5): 1174–1189. doi:10.1016/j.jmb.2008.09.059.
- ^ Colović MB, Krstić DZ, Lazarević-Pašti TD, Bondžić AM, Vasić VM (May 2013). "Acetylcholinesterase inhibitors: pharmacology and toxicology". Current Neuropharmacology. 11 (3): 315–35. doi:10.2174/1570159X11311030006. PMC 3648782. PMID 24179466.
- ^ Oeser, Petr; Tobrman, Tomáš (2 April 2024). "Organophosphates as Versatile Substrates in Organic Synthesis". Molecules. 29 (7): 1593. doi:10.3390/molecules29071593.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Metcalf, William W.; van der Donk, Wilfred A. (1 June 2009). "Biosynthesis of Phosphonic and Phosphinic Acid Natural Products". Annual Review of Biochemistry. 78 (1): 65–94. doi:10.1146/annurev.biochem.78.091707.100215.
- ^ Liu, Yaxin; Gong, Shuai; Ye, Langjie; Li, Jianhua; Liu, Chunsheng; Chen, Da; Fang, Mingliang; Letcher, Robert J.; Su, Guanyong (October 2021). "Organophosphate (OP) diesters and a review of sources, chemical properties, environmental occurrence, adverse effects, and future directions". Environment International. 155: 106691. doi:10.1016/j.envint.2021.106691.
- ^ Arora, Pinklesh; Singh, Rakhi; Seshadri, Geetha; Tyagi, Ajay Kumar (16 July 2018). "Synthesis, Properties and Applications of Anionic Phosphate Ester Surfactants: A Review". Tenside Surfactants Detergents. 55 (4): 266–272. doi:10.3139/113.110570.
- ^ Tracy, David J.; Reierson, Robert L. (April 2002). "Commercial synthesis of monoalkyl phosphates". Journal of Surfactants and Detergents. 5 (2): 169–172. doi:10.1007/s11743-002-0218-9.
- ^ Arora, Pinklesh; Singh, Rakhi; Seshadri, Geetha; Tyagi, Ajay Kumar (16 July 2018). "Synthesis, Properties and Applications of Anionic Phosphate Ester Surfactants: A Review". Tenside Surfactants Detergents. 55 (4): 266–272. doi:10.3139/113.110570.
- ^ Liu, Runzeng; Mabury, Scott A. (19 February 2019). "Organophosphite Antioxidants in Indoor Dust Represent an Indirect Source of Organophosphate Esters". Environmental Science & Technology. 53 (4): 1805–1811. doi:10.1021/acs.est.8b05545.
- ^ Bi, Ruifeng; Meng, Weikun; Su, Guanyong (July 2023). "Organophosphate esters (OPEs) in plastic food packaging: non-target recognition, and migration behavior assessment". Environment International. 177: 108010. doi:10.1016/j.envint.2023.108010.
- ^ Fugel, Malte; Malaspina, Lorraine A.; Pal, Rumpa; Thomas, Sajesh P.; Shi, Ming W.; Spackman, Mark A.; Sugimoto, Kunihisa; Grabowsky, Simon (7 May 2019). "Revisiting a Historical Concept by Using Quantum Crystallography: Are Phosphate, Sulfate and Perchlorate Anions Hypervalent?". Chemistry – A European Journal. 25 (26): 6523–6532. doi:10.1002/chem.201806247.
- ^ Cundari, Thomas R. (2013). "Chemical bonding involving d-orbitals". Chemical Communications. 49 (83): 9521. doi:10.1039/c3cc45204b.
- ^ Magnusson, Eric (October 1990). "Hypercoordinate molecules of second-row elements: d functions or d orbitals?". Journal of the American Chemical Society. 112 (22): 7940–7951. doi:10.1021/ja00178a014.
- ^ a b Gamoke, Benjamin; Neff, Diane; Simons, Jack (14 May 2009). "Nature of PO Bonds in Phosphates". The Journal of Physical Chemistry A. 113 (19): 5677–5684. doi:10.1021/jp810014s.
- ^ Rajani, Puchakayala; Gopakumar, Gopinadhanpillai; Nagarajan, Sivaraman; Brahmmananda Rao, Cherukuri Venkata Siva (July 2021). "Does the basicity of phosphoryl oxygen change with alkyl chain length in phosphate ligands?". Chemical Physics Letters. 775: 138641. doi:10.1016/j.cplett.2021.138641.
- ^ Chesnut, D. B. (1 May 2003). "Atoms-in-Molecules and Electron Localization Function Study of the Phosphoryl Bond". The Journal of Physical Chemistry A. 107 (21): 4307–4313. doi:10.1021/jp022292r.
- ^ Corbridge, Derek E. C. (1971). "The structural chemistry of phosphates". Bulletin de la Société française de Minéralogie et de Cristallographie. 94 (3): 271–299. doi:10.3406/bulmi.1971.6534.
- ^ Rai, Uma S.; Symons, Martyn C. R. (1994). "EPR data do not support the P=O representation for trialkyl phosphates and phosphine oxides or sulfides". J. Chem. Soc., Faraday Trans. 90 (18): 2649–2652. doi:10.1039/FT9949002649.
- ^ Kumler, W. D.; Eiler, John J. (December 1943). "The Acid Strength of Mono and Diesters of Phosphoric Acid. The n-Alkyl Esters from Methyl to Butyl, the Esters of Biological Importance, and the Natural Guanidine Phosphoric Acids". Journal of the American Chemical Society. 65 (12): 2355–2361. doi:10.1021/ja01252a028.
- ^ Möller, A.; Sturm, R.; Xie, Z.; Cai, M.; He, J.; Ebinghaus, R. (2012). "Organophosphorus Flame Retardants and Plasticizers in Airborne Particles over the Northern Pacific and Indian Ocean toward the Polar Regions: Evidence for Global Occurrence". Environmental Science and Technology. 46 (6): 3127–3134. Bibcode:2012EnST...46.3127M. doi:10.1021/es204272v. PMID 22332897.
- ^ McDonough, Carrie A.; De Silva, Amila O.; Sun, Caoxin; Cabrerizo, Ana; Adelman, David; Soltwedel, Thomas; Bauerfeind, Eduard; Muir, Derek C. G.; Lohmann, Rainer (2018-06-05). "Dissolved Organophosphate Esters and Polybrominated Diphenyl Ethers in Remote Marine Environments: Arctic Surface Water Distributions and Net Transport through Fram Strait". Environmental Science & Technology. 52 (11): 6208–6216. Bibcode:2018EnST...52.6208M. doi:10.1021/acs.est.8b01127. ISSN 0013-936X. PMID 29787253.
- ^ "Status and Trends of Pesticide Use". United Nations Environment Programme. World Health Organization, & Food and Agriculture Organization of the United Nations. 2022.
- ^ a b c van den Berg, Henk; da Silva Bezerra, Haroldo Sergio; Al-Eryani, Samira; Chanda, Emmanuel; Nagpal, Bhupender N.; Knox, Tessa B.; Velayudhan, Raman; Yadav, Rajpal S. (13 December 2021). "Recent trends in global insecticide use for disease vector control and potential implications for resistance management". Scientific Reports. 11 (1). doi:10.1038/s41598-021-03367-9.
- ^ a b Costa, Lucio G (1 March 2018). "Organophosphorus Compounds at 80: Some Old and New Issues". Toxicological Sciences. 162 (1): 24–35. doi:10.1093/toxsci/kfx266.
- ^ Ritter SK (2009). "Pinpointing Trends In Pesticide Use. Limited data indicate that pesticide use has dropped since the 1970s". Chemical & Engineering News. Vol. 87, no. 7. ACS. ISSN 0009-2347.
- ^ Costa, Lucio G. (1987). "Toxicology of Pesticides: A Brief History". Toxicology of Pesticides: 1–10. doi:10.1007/978-3-642-70898-5_1.
- ^ Richmond, Martha (2021). "Discovery and Commercial Introduction and Mode of Action of Parathion, Malathion, Diazinon, Tetrachlorvinphos, and Glyphosate". Cancer Hazards: Parathion, Malathion, Diazinon, Tetrachlorvinphos and Glyphosate: 3–11. doi:10.1007/978-3-030-81953-8_1.
- ^ Peter, J. V.; Sudarsan, T. I.; Moran, J. L. (2014). "Clinical features of organophosphate poisoning: A review of different classification systems and approaches". Indian Journal of Critical Care Medicine. 18 (11): 735–745. doi:10.4103/0972-5229.144017. PMC 4238091. PMID 25425841.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ O’Brien, R. D.; Thorn, G. D.; Fisher, R. W. (1 October 1958). "New Organophosphate Insecticides Developed on Rational Principles1". Journal of Economic Entomology. 51 (5): 714–718. doi:10.1093/jee/51.5.714.
- ^ Salgado, Vincent L; David, Michael D (April 2017). "Chance and design in proinsecticide discovery". Pest Management Science. 73 (4): 723–730. doi:10.1002/ps.4502.
- ^ Gage, J. C. (1 June 1953). "A cholinesterase inhibitor derived from OO -diethyl O - p -nitrophenyl thiophosphate in vivo". Biochemical Journal. 54 (3): 426–430. doi:10.1042/bj0540426.
- ^ "The decomposition of some organophosphorus insecticides and related compounds in plants". Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences. 239 (663): 191–214. 22 December 1955. doi:10.1098/rstb.1955.0009.
- ^ Spencer, E. Y.; O'Brien, R. D.; White, R. W. (February 1957). "Metabolism of Insecticides, Permanganate Oxidation Products of Schradan". Journal of Agricultural and Food Chemistry. 5 (2): 123–127. doi:10.1021/jf60072a004.
- ^ Davison, A. N. (1 June 1955). "Return of cholinesterase activity in the rat after inhibition by organophosphorus compounds. 2. A comparative study of true and pseudo cholinesterase". Biochemical Journal. 60 (2): 339–346. doi:10.1042/bj0600339.
- ^ Metcalf, Robert L.; March, Ralph B. (1 March 1953). "Further Studies1 on the Mode of Action of Organic Thionophosphate Insecticides". Annals of the Entomological Society of America. 46 (1): 63–74. doi:10.1093/aesa/46.1.63.
- ^ Spencer, E. Y.; O'Brien, R. D. (August 1953). "Schradan, Enhancement of Anticholinesterase Activity in Octamethylpyrophosphoramide by Chlorine". Journal of Agricultural and Food Chemistry. 1 (11): 716–720. doi:10.1021/jf60011a003.
- ^ O'Brien, R. D. (1 May 1961). "The effect of SKF 525A (2-diethylaminoethyl 2:2-diphenylvalerate hydrochloride) on organophosphate metabolism in insects and mammals". Biochemical Journal. 79 (2): 229–235. doi:10.1042/bj0790229.
- ^ Mew, Emma J.; Padmanathan, Prianka; Konradsen, Flemming; Eddleston, Michael; Chang, Shu-Sen; Phillips, Michael R.; Gunnell, David (September 2017). "The global burden of fatal self-poisoning with pesticides 2006-15: Systematic review". Journal of Affective Disorders. 219: 93–104. doi:10.1016/j.jad.2017.05.002.
- ^ Jokanović, Milan; Oleksak, Patrik; Kuca, Kamil (January 2023). "Multiple neurological effects associated with exposure to organophosphorus pesticides in man". Toxicology. 484: 153407. doi:10.1016/j.tox.2022.153407.
- ^ "The environmental, human health and economic impacts of pesticides" (PDF). United Nations Environment Programme [UNEP]. Retrieved 2 January 2024.
- ^ "Some Organophosphate Insecticides and Herbicides". IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. 12. International Agency for Research on Cancer: 464. 2017. PMID 31829533.
- ^ Muñoz-Quezada, María Teresa; Lucero, Boris A.; Barr, Dana B.; Steenland, Kyle; Levy, Karen; Ryan, P. Barry; Iglesias, Veronica; Alvarado, Sergio; Concha, Carlos; Rojas, Evelyn; Vega, Catalina (December 2013). "Neurodevelopmental effects in children associated with exposure to organophosphate pesticides: A systematic review". NeuroToxicology. 39: 158–168. doi:10.1016/j.neuro.2013.09.003.
- ^ Muñoz-Quezada, María Teresa; Lucero, Boris Andrés; Iglesias, Verónica Paz; Muñoz, María Pía; Cornejo, Claudia Alejandra; Achu, Eduardo; Baumert, Brittney; Hanchey, Arianna; Concha, Carlos; Brito, Ana María; Villalobos, Marcos (2 January 2016). "Chronic exposure to organophosphate (OP) pesticides and neuropsychological functioning in farm workers: a review". International Journal of Occupational and Environmental Health. 22 (1): 68–79. doi:10.1080/10773525.2015.1123848.
- ^ "Status and Trends of Pesticide Use". United Nations Environment Programme. World Health Organization, & Food and Agriculture Organization of the United Nations. 2022.
- ^ Clune, Alison L.; Ryan, P. Barry; Barr, Dana Boyd (April 2012). "Have Regulatory Efforts to Reduce Organophosphorus Insecticide Exposures Been Effective?". Environmental Health Perspectives. 120 (4): 521–525. doi:10.1289/ehp.1104323.
- ^ Stone, David L; Sudakin, Daniel L; Jenkins, Jeffrey J (December 2009). "Longitudinal trends in organophosphate incidents reported to the National Pesticide Information Center, 1995–2007". Environmental Health. 8 (1). doi:10.1186/1476-069X-8-18.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Souza, Marília Cristina Oliveira; Cruz, Jonas Carneiro; Cesila, Cibele Aparecida; Gonzalez, Neus; Rocha, Bruno Alves; Adeyemi, Joseph A.; Nadal, Marti; Domingo, José L.; Barbosa, Fernando (July 2023). "Recent trends in pesticides in crops: A critical review of the duality of risks-benefits and the Brazilian legislation issue". Environmental Research. 228: 115811. doi:10.1016/j.envres.2023.115811.
- ^ Galt, Ryan E. (October 2008). "Beyond the circle of poison: Significant shifts in the global pesticide complex, 1976–2008" (PDF). Global Environmental Change. 18 (4): 786–799. doi:10.1016/j.gloenvcha.2008.07.003.
- ^ Maggi, Federico; Tang, Fiona H. M.; la Cecilia, Daniele; McBratney, Alexander (12 September 2019). "PEST-CHEMGRIDS, global gridded maps of the top 20 crop-specific pesticide application rates from 2015 to 2025". Scientific Data. 6 (1). doi:10.1038/s41597-019-0169-4.
- ^ "Tolerance Revocations: Chlorpyrifos". www.regulations.gov. Retrieved 2 January 2024.
Federal Register Number: 2021-18091
- ^ Umetsu, Noriharu; Shirai, Yuichi (20 May 2020). "Development of novel pesticides in the 21st century". Journal of Pesticide Science. 45 (2): 54–74. doi:10.1584/jpestics.D20-201.
- ^ Gray, George M.; Hammitt, James K. (October 2000). "Risk/Risk Trade‐offs in Pesticide Regulation: An Exploratory Analysis of the Public Health Effects of a Ban on Organophosphate and Carbamate Pesticides". Risk Analysis. 20 (5): 665–680. doi:10.1111/0272-4332.205060.
- ^ Siegfried, Blair D.; Scharf, Michael E. (2001). "Mechanisms of Organophosphate Resistance in Insects". Biochemical Sites of Insecticide Action and Resistance: 269–291. doi:10.1007/978-3-642-59549-3_13.
- ^ a b van der Veen, Ike; de Boer, Jacob (August 2012). "Phosphorus flame retardants: Properties, production, environmental occurrence, toxicity and analysis". Chemosphere. 88 (10): 1119–1153. doi:10.1016/j.chemosphere.2012.03.067.
- ^ Schmitt, Elmar (May 2007). "Phosphorus-based flame retardants for thermoplastics". Plastics, Additives and Compounding. 9 (3): 26–30. doi:10.1016/S1464-391X(07)70067-3.
- ^ He, Huan; Gao, Zhanqi; Zhu, Donglin; Guo, Jiehong; Yang, Shaogui; Li, Shiyin; Zhang, Limin; Sun, Cheng (December 2017). "Assessing bioaccessibility and bioavailability of chlorinated organophosphorus flame retardants in sediments". Chemosphere. 189: 239–246. doi:10.1016/j.chemosphere.2017.09.017.
- ^ Blum, Arlene; Behl, Mamta; Birnbaum, Linda S.; Diamond, Miriam L.; Phillips, Allison; Singla, Veena; Sipes, Nisha S.; Stapleton, Heather M.; Venier, Marta (12 November 2019). "Organophosphate Ester Flame Retardants: Are They a Regrettable Substitution for Polybrominated Diphenyl Ethers?". Environmental Science & Technology Letters. 6 (11): 638–649. doi:10.1021/acs.estlett.9b00582.
- ^ Du, Jia; Li, Huanxuan; Xu, Shaodan; Zhou, Qingwei; Jin, Meiqing; Tang, Junhong (August 2019). "A review of organophosphorus flame retardants (OPFRs): occurrence, bioaccumulation, toxicity, and organism exposure". Environmental Science and Pollution Research. 26 (22): 22126–22136. doi:10.1007/s11356-019-05669-y.
- ^ "ECHA identifies certain brominated flame retardants as candidates for restriction". echa.europa.eu. European Chemicals Agency. Retrieved 3 January 2024.
- ^ a b "Regulatory strategy for flame retardant". European Chemicals Agency. Retrieved 3 January 2024.doi:10.2823/854233
- ^ Weil, E.D.; Levchik, S.V. (26 January 2001). "Phosphorus Flame Retardants". Kirk-Othmer Encyclopedia of Chemical Technology. doi:10.1002/0471238961.1608151923050912.a01.pub3.
- ^ Pawlowski, Kristin H; Schartel, Bernhard (November 2007). "Flame retardancy mechanisms of triphenyl phosphate, resorcinol bis(diphenyl phosphate) and bisphenol A bis(diphenyl phosphate) in polycarbonate/acrylonitrile–butadiene–styrene blends". Polymer International. 56 (11): 1404–1414. doi:10.1002/pi.2290.
- ^ Blum, Arlene; Behl, Mamta; Birnbaum, Linda S.; Diamond, Miriam L.; Phillips, Allison; Singla, Veena; Sipes, Nisha S.; Stapleton, Heather M.; Venier, Marta (12 November 2019). "Organophosphate Ester Flame Retardants: Are They a Regrettable Substitution for Polybrominated Diphenyl Ethers?". Environmental Science & Technology Letters. 6 (11): 638–649. doi:10.1021/acs.estlett.9b00582.
- ^ Du, Jia; Li, Huanxuan; Xu, Shaodan; Zhou, Qingwei; Jin, Meiqing; Tang, Junhong (August 2019). "A review of organophosphorus flame retardants (OPFRs): occurrence, bioaccumulation, toxicity, and organism exposure". Environmental Science and Pollution Research. 26 (22): 22126–22136. doi:10.1007/s11356-019-05669-y.
- ^ Barth, Mary L.; Craig, Peter H. (October 1999). "Evaluation of the hazards of industrial exposure to tricresyl phosphate:a review and interpretation of the literature". Journal of Toxicology and Environmental Health, Part B. 2 (4): 281–300. doi:10.1080/109374099281142.
- ^ Truong, Jimmy W.; Diamond, Miriam L.; Helm, Paul A.; Jantunen, Liisa M. (December 2017). "Isomers of tris(chloropropyl) phosphate (TCPP) in technical mixtures and environmental samples". Analytical and Bioanalytical Chemistry. 409 (30): 6989–6997. doi:10.1007/s00216-017-0572-7.
- ^ Amiri, Roshanak; Bissram, Meera J.; Hashemihedeshi, Mahin; Dorman, Frank L.; Megson, David; Jobst, Karl J. (5 April 2023). "Differentiating Toxic and Nontoxic Tricresyl Phosphate Isomers Using Ion–Molecule Reactions with Oxygen". Journal of the American Society for Mass Spectrometry. 34 (4): 640–648. doi:10.1021/jasms.2c00334.
- ^ Duarte, Daniel J.; Rutten, Joost M.M.; van den Berg, Martin; Westerink, Remco H.S. (March 2017). "In vitro neurotoxic hazard characterization of different tricresyl phosphate (TCP) isomers and mixtures". NeuroToxicology. 59: 222–230. doi:10.1016/j.neuro.2016.02.001.
- ^ a b Cadogan DF, Howick CJ (15 June 2000). "Plasticizers". Ullmann's Encyclopedia of Industrial Chemistry. 27: 613–614. doi:10.1002/14356007.a20_439. ISBN 3527306730.
- ^ Rahman, M; Brazel, C (December 2004). "The plasticizer market: an assessment of traditional plasticizers and research trends to meet new challenges". Progress in Polymer Science. 29 (12): 1223–1248. doi:10.1016/j.progpolymsci.2004.10.001.
- ^ Krauskopf LG (2009). "3.13 Plasticizers". Plastics additives handbook (6. ed.). Munich: Carl Hanser Verlag. pp. 485–511. ISBN 978-3-446-40801-2.
- ^ William Coaker, A. (September 2003). "Fire and flame retardants for PVC". Journal of Vinyl and Additive Technology. 9 (3): 108–115. doi:10.1002/vnl.10072.
- ^ Grossman, Richard F (2008-05-02). Handbook of Vinyl Formulating. John Wiley & Sons. p. 289. ISBN 978-0-470-25354-0.
- ^ Levchik, Sergei V.; Weil, Edward D. (October 2005). "Overview of the recent literature on flame retardancy and smoke suppression in PVC". Polymers for Advanced Technologies. 16 (10): 707–716. doi:10.1002/pat.645.
- ^ Rudnick, L.R. (2013). "Chapter 4: Neutral Phosphate Esters". Synthetics, Mineral Oils, and Bio-Based Lubricants: Chemistry and Technology, Second Edition. Chemical Industries. CRC Press. pp. 81–104. doi:10.1201/9781315158150-4. ISBN 978-1-4398-5538-6. Retrieved 2024-01-07.
- ^ Guan, Bihan; Pochopien, Bernadeta A.; Wright, Dominic S. (August 2016). "The chemistry, mechanism and function of tricresyl phosphate (TCP) as an anti‐wear lubricant additive". Lubrication Science. 28 (5): 257–265. doi:10.1002/ls.1327.
- ^ Johnson, David; Hils, John (18 December 2013). "Phosphate Esters, Thiophosphate Esters and Metal Thiophosphates as Lubricant Additives". Lubricants. 1 (4): 132–148. doi:10.3390/lubricants1040132.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Li, Haogang; Zhang, Yanbin; Li, Changhe; Zhou, Zongming; Nie, Xiaolin; Chen, Yun; Cao, Huajun; Liu, Bo; Zhang, Naiqing; Said, Zafar; Debnath, Sujan; Jamil, Muhammad; Ali, Hafiz Muhammad; Sharma, Shubham (May 2022). "Extreme pressure and antiwear additives for lubricant: academic insights and perspectives". The International Journal of Advanced Manufacturing Technology. 120 (1–2): 1–27. doi:10.1007/s00170-021-08614-x.
- ^ Hidayah, Nur Nadiatul; Abidin, Sumaiya Zainal (June 2018). "The evolution of mineral processing in extraction of rare earth elements using liquid-liquid extraction: A review". Minerals Engineering. 121: 146–157. doi:10.1016/j.mineng.2018.03.018.
- ^ Xie, Feng; Zhang, Ting An; Dreisinger, David; Doyle, Fiona (February 2014). "A critical review on solvent extraction of rare earths from aqueous solutions". Minerals Engineering. 56: 10–28. doi:10.1016/j.mineng.2013.10.021.
- ^ Paiva, A. P.; Malik, P. (2004). "Recent advances on the chemistry of solvent extraction applied to the reprocessing of spent nuclear fuels and radioactive wastes". Journal of Radioanalytical and Nuclear Chemistry. 261 (2): 485–496. doi:10.1023/B:JRNC.0000034890.23325.b5. S2CID 94173845.
- ^ a b Farn, R.J. (2008). Chemistry and Technology of Surfactants. Wiley. pp. 122–124. ISBN 978-1-4051-7179-3. Retrieved 2023-05-27.
- ^ Arora, Pinklesh; Singh, Rakhi; Seshadri, Geetha; Tyagi, Ajay Kumar (16 July 2018). "Synthesis, Properties and Applications of Anionic Phosphate Ester Surfactants: A Review". Tenside Surfactants Detergents. 55 (4): 266–272. doi:10.3139/113.110570.
- ^ Kaneko, T.M.; Spicer, L.D. (1985). Pesticide Formulations and Application Systems: Fourth Symposium : a Symposium Sponsored by ASTM Committee E-35 on Pesticides, New Orleans, La., 2-3 Nov. 1983. ASTM special technical publication. ASTM. pp. 5–14. ISBN 978-0-8031-0413-6. Retrieved 2023-05-27.
- ^ Miller, Dennis; Wiener, Eva-Maria; Turowski, Angelika; Thunig, Christine; Hoffmann, Heinz (July 1999). "O/W emulsions for cosmetics products stabilized by alkyl phosphates — rheology and storage tests". Colloids and Surfaces A: Physicochemical and Engineering Aspects. 152 (1–2): 155–160. doi:10.1016/S0927-7757(98)00630-X.
- ^ Petroianu, G. A. (2010-10-01). "Toxicity of phosphor esters: Willy Lange (1900-1976) and Gerda von Krueger (1907-after 1970)". Die Pharmazie. 65 (10): 776–780. ISSN 0031-7144. PMID 21105582.
- ^ Chai, Peter R.; Hayes, Bryan D.; Erickson, Timothy B.; Boyer, Edward W. (January 2018). "Novichok agents: a historical, current, and toxicological perspective". Toxicology Communications. 2 (1): 45–48. doi:10.1080/24734306.2018.1475151. PMC 6039123. PMID 30003185. S2CID 49661943.
- ^ Vale, J. Allister; Marrs, Timothy C.; Maynard, Robert L. (2 November 2018). "Novichok: a murderous nerve agent attack in the UK". Clinical Toxicology. 56 (11): 1093–1097. doi:10.1080/15563650.2018.1469759.
- ^ Westheimer, F. H. (6 March 1987). "Why Nature Chose Phosphates". Science. 235 (4793): 1173–1178. doi:10.1126/science.2434996.
- ^ Kamerlin, Shina C. L.; Sharma, Pankaz K.; Prasad, Ram B.; Warshel, Arieh (February 2013). "Why nature really chose phosphate". Quarterly Reviews of Biophysics. 46 (1): 1–132. doi:10.1017/S0033583512000157.
- ^ Salamova, Amina; Ma, Yuning; Venier, Marta; Hites, Ronald A. (2014-01-14). "High Levels of Organophosphate Flame Retardants in the Great Lakes Atmosphere". Environmental Science & Technology Letters. 1 (1): 8–14. doi:10.1021/ez400034n.
- ^ a b Venier, Marta; Dove, Alice; Romanak, Kevin; Backus, Sean; Hites, Ronald (2014-08-19). "Flame Retardants and Legacy Chemicals in Great Lakes' Water". Environmental Science & Technology. 48 (16): 9563–9572. Bibcode:2014EnST...48.9563V. doi:10.1021/es501509r. ISSN 0013-936X. PMID 25045802.